Theoretical Study on the Aggregation of Copper Clusters on a Liquid Surface
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Copper Clusters Cun (n = 2–80)
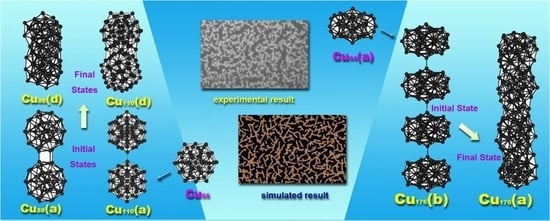
3.2. Aggregation of the Cun Clusters
3.3. Anisotropic Interaction Between Two Cu Clusters
3.4. Simulation of the Aggregation of Copper Clusters on a Liquid Surface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ling, D.S.; Lee, N.; Hyeon, T. Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical application. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Dou, X.Y.; Chen, X.Y.; Zhu, H.G.; Liu, Y.; Chen, D.Y.; Yuan, X.; Yao, Q.F.; Xie, J.P. Water-soluble metal nanoculsters: Recent advances in molecular-level exploration and biomedical applications. Dalton Trans. 2019, 48, 10385–10392. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.N.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Zeng, Z.P.; Liu, B. Bridging the gap: Electron relay and plasmonic sentization of metal nanocrystals for metal clusters. J. Am. Chem. Soc. 2015, 137, 10735–10744. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Chen, R.H.; Zhang, X.M.; Chen, F.J.; Yan, J.Z.; Sun, C.F.; Ou, D.H.; Peng, J.; Lin, S.C.; Tang, Z.C.; et al. Ether-soluble Cu53 nanoclusters as an effective precursor of high-quality cul films for optoelectronic applications. Angew. Chem. Int. Ed. 2019, 58, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Die, D.; Zheng, B.X.; Kuang, X.Y.; Zhao, Z.Q.; Guo, J.H.; Du, Q. Exploration of the structural, electronic and tunable magnetic properties of Cu4M (M = Sc-Ni) Clusters. Materials 2017, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Li, Q.J.; Si, R.; Li, G.D.; Li, W.; Liu, D.P.; Wang, D.J.; Sun, L.; Zhang, Y.; Zou, X.X. Coupling sub-nanometric copper clusters with quasi-amorphous cobalt sulfide yields efficient and robust electrocatalysts for water splitting reaction. Adv. Mater. 2017, 29, 1606200. [Google Scholar] [CrossRef]
- Li, C.G.; Yuan, Y.Q.; Hu, Y.F.; Zhang, J.; Tang, Y.N.; Ren, B.Z. Density function theory study of the structures and electronic properties of copper and sulfur doped copper clusters. Comput. Theor. Chem. 2016, 1080, 47–55. [Google Scholar] [CrossRef]
- Liu, C.; Yang, B.; Tyo, E.; Seifert, S.; DeBartolo, J.; Issendorff, B.V.; Zapol, P.; Vajda, S.; Curtiss, L.A. Carbon dioxide conversion to methanol over size-selected Cu4 clusters at low pressures. J. Am. Chem. Soc. 2015, 137, 8676–8679. [Google Scholar] [CrossRef]
- Yang, B.; Liu, C.; Halder, A.; Tyo, E.C.; Martinson, A.B.F.; Seifert, S.; Zapol, P.; Curtiss, L.A.; Vajda, S. Copper cluster size effect in methanol synthesis from CO2. J. Phys. Chem. C 2017, 121, 10406–10412. [Google Scholar] [CrossRef]
- Delley, B.; Ellis, D.E.; Freeman, A.J.; Baerends, E.J.; Post, D. Binding energy and electronic structure of small copper particles. Phys. Rev. B 1983, 27, 2132. [Google Scholar] [CrossRef]
- Christensen, O.B.; Jacobsen, K.W.; Nørskov, J.K.; Manninen, M. Cu cluster shell structure at elevated temperatures. Phys. Rev. Lett. 1991, 66, 2219. [Google Scholar] [CrossRef] [PubMed]
- Doye, J.P.K.; Wales, D.J. Global minima for transition metal clusters described by Sutton-Chen potentials. New J. Chem. 1998, 22, 733. [Google Scholar] [CrossRef]
- Sutton-Chen Clusters. Available online: http://doye.chem.ox.ac.uk/jon/structures/SC.html (accessed on 24 November 2019).
- Luo, Z.X.; Castleman, A.W.; Khanna, S.N. Reactivity of metal clusters. Chem. Rev. 2016, 116, 14456–14492. [Google Scholar] [CrossRef] [PubMed]
- Jaque, P.; Toro-Labbé, A. Characterization of copper clusters through the use of density functional theory reactivity descriptors. J. Chem. Phys. 2002, 117, 3208. [Google Scholar] [CrossRef]
- Darby, S.; Mortimer-Jones, T.V.; Johnston, R.L.; Roberts, C. Theoretical study of Cu-Au nanoalloy clusters using a genetic algorithm. J. Chem. Phys. 2002, 116, 1536. [Google Scholar] [CrossRef]
- Tang, Q.; Lee, Y.J.; Li, D.Y.; Choi, W.J.; Lee, D.; Jiang, D.E. Lattice-hydride mechanism in electrocatalytic CO2 reduction by structurally precise copper-hydride nanoclusters. J. Am. Chem. Soc. 2017, 139, 9728–9736. [Google Scholar] [CrossRef]
- Kabir, M.; Mookerjee, A.; Bhattacharya, A.K. Structure and stability of copper clusters: A tight-binding molecular dynamics study. Phys. Rev. A 2004, 69, 043203. [Google Scholar] [CrossRef]
- Grigoryan, V.G.; Alamanova, D.; Springborg, M. Structure and energetics of CuN clusters with (2 ≤ N ≤ 150): An embedded-atom-method study. Phys. Rev. B 2006, 73, 115415. [Google Scholar] [CrossRef]
- Zhang, M.L.; Li, G.P. Energy and Structure of Copper Clusters (n = 2–70,147,500) Studied by the Monte Carlo Method. Solid State Phenom. 2007, 121, 607–610. [Google Scholar] [CrossRef]
- Pan, X.D.; Gai, Z.G.; Gong, P.L. Atomic and molecular physics: Energy and structure of copper clusters (n = 70–150) studied by the Monte Carlo computer simulation. Chin. Phys. B 2008, 17, 3329. [Google Scholar]
- Chen, M.G.; Xie, J.P.; Ye, G.X. Formation mechanism and ordered patterns in Cu films deposited on silicone oil surfaces. Phys. Lett. A 2006, 360, 323–326. [Google Scholar] [CrossRef]
- Ye, G.X.; Zhang, Q.R.; Feng, C.M.; Ge, H.L.; Jiao, Z.K. Structural and electrical properties of a metallic rough thin film system deposited on liquid substrates. Phys. Rev. B 1996, 54, 14754. [Google Scholar] [CrossRef] [PubMed]
- Wender, H.; De Oliveira, L.F.; Feil, A.F.; Lissner, E.; Migowski, P.; Meneghetti, M.R.; Teixeira, S.R.; Dupont, J. Synthesis of gold nanoparticles in a biocompatible fluid from sputtering deposition onto castor oil. Chem. Commun. 2010, 46, 7019–7021. [Google Scholar] [CrossRef]
- Yoshida, H.; Kawamoto, K.; Kubo, H.; Tsuda, T.; Fujii, A.; Kuwabata, S.; Ozaki, M. Nanoparticle-Dispersed Liquid Crystals Fabricated by Sputter Doping. Adv. Mater. 2010, 22, 622–626. [Google Scholar] [CrossRef]
- Pan, Q.F.; Cheng, Y.; Tao, X.M.; Yang, B.; Li, B.X.; Ye, G.X. Temperature Dependence of the Aggregation Behavior of Aluminum Nanoparticles on Liquid Substrate. J. Nanopart. Res. 2015, 17, 161. [Google Scholar] [CrossRef]
- Lu, C.X.; Jin, Y.; Tao, X.M.; Yang, B.; Ye, G.X. Nucleation and growth of zinc crystals on a liquid surface. CrystEngComm 2018, 20, 122–127. [Google Scholar] [CrossRef]
- ADF2010.02, SCF. Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Lenthe, E.V.; Baerends, E.J. Optimized slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef]
- Brandbyge, M.; Mozos, J.L.; Ordejon, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165401. [Google Scholar] [CrossRef]
- Taylor, J.; Guo, H.; Wang, J. Ab initio modeling of quantum transport properties of molecular electronic devices. Phys. Rev. B 2001, 63, 245407. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Kawazoe, Y. Hund’s rule in metal clusters: Prediction of high magnetic moment state of Al12Cu from first-principles calculations. Phys. Rev. B 2001, 64, 115405. [Google Scholar] [CrossRef]
- Die, D.; Zheng, B.X.; Zhao, L.Q.; Zhu, Q.W.; Zhao, Z.Q. Insights into the structural, electronic and magnetic properties of V-doped copper clusters: Comparison with pure copper clusters. Sci. Rep. 2016, 6, 31978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulbazine, M.; Boudjahem, A.G. Stability, Electronic and Magnetic Properties of Mn-Doped Copper Clusters: A Meta-GGA Functional Investigation. J. Clust. Sci. 2019, 30, 31–44. [Google Scholar] [CrossRef]
- Spasov, V.A.; Lee, T.H.; Ervin, K.M. Threshold collision-induced dissociation of anionic copper clusters and copper cluster monocarbonyls. J. Chem. Phys. 2000, 112, 1713–1720. [Google Scholar] [CrossRef]
- Tamijani, A.A.; Salam, A.; Lara-Castells, M.P. Adsorption of Noble-Gas Atoms on the TiO2(110) Surface: An Ab Initio-Assisted Study with van der Waals-Corrected DFT. J. Phys. Chem. C 2016, 120, 18126. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Berland, K.; Cooper, V.R.; Lee, K.; Schröder, E.; Thonhauser, T.; Hyldgaard, P.; Lundqvist, B.I. van der Waals forces in density functional theory: A review of the vdW-DF method. Rep. Prog. Phys. 2015, 78, 066501. [Google Scholar] [CrossRef] [Green Version]








| Cun | BE | Cun | BE | Cun | BE | Cun | BE | Cun | BE |
|---|---|---|---|---|---|---|---|---|---|
| Cu2 | 2.47 | Cu16 | 35.17 | Cu29 | 68.08 | Cu45 | 111.02 | Cu63 | 160.52 |
| 2.58 | 41.79 | 81.32 | 133.22 | 193.04 | |||||
| Cu3 | 3.69 | Cu17 | 38.18 | Cu30 | 71.09 | Cu46 | 114.81 | Cu64 | 163.45 |
| 4.00 | 45.23 | 85.05 | 137.89 | 196.63 | |||||
| 3.21 * | Cu18 | 40.98 | Cu31(a) | 74.11 | Cu47 | 117.46 | Cu65 | 165.98 | |
| Cu4 | 4.88 | 48.47 | 88.58 | 141.03 | 199.82 | ||||
| 5.68 | Cu19 | 43.14 | Cu31(b) | 74.14 | Cu48 | 119.50 | Cu66 | 169.07 | |
| 5.92 * | 51.21 | 88.52 | 143.53 | 203.19 | |||||
| Cu5 | 7.89 | Cu20 | 45.89 | Cu32 | 76.82 | Cu49 | 123.33 | Cu67 | 171.52 |
| 9.00 | 54.40 | 91.83 | 148.18 | 206.57 | |||||
| 7.80 * | Cu21 | 48.26 | Cu33 | 79.67 | Cu50 | 125.15 | Cu68 | 174.76 | |
| Cu6 | 9.91 | 57.29 | 95.14 | 150.33 | 209.99 | ||||
| 11.80 | Cu22 | 50.32 | Cu34 | 82.36 | Cu51 | 127.93 | Cu69 | 176.76 | |
| 10.38 * | 59.91 | 98.26 | 154.00 | 212.87 | |||||
| Cu7 | 13.40 | Cu23(a) | 53.14 | Cu35 | 85.26 | Cu52 | 131.33 | Cu70 | 179.57 |
| 15.33 | 63.30 | 101.83 | 157.99 | 216.22 | |||||
| 13.02 * | Cu23(b) | 52.80 | Cu36 | 87.58 | Cu53 | 134.57 | Cu71 | 182.38 | |
| Cu8 | 16.06 | 63.04 | 104.81 | 161.90 | 219.77 | ||||
| 18.47 | Cu24(a) | 55.62 | Cu37 | 90.26 | Cu54 | 137.97 | Cu72 | 185.20 | |
| 16.00 * | 66.07 | 108.04 | 165.85 | 223.11 | |||||
| Cu9 | 17.89 | Cu24(b) | 55.00 | Cu38 | 93.08 | Cu55 | 141.24 | Cu73 | 187.97 |
| 20.77 | 65.63 | 111.53 | 169.80 | 226.41 | |||||
| Cu10 | 19.83 | Cu25(a) | 57.77 | Cu39 | 95.66 | Cu56 | 143.81 | Cu74 | 190.55 |
| 23.45 | 69.01 | 114.50 | 172.75 | 229.72 | |||||
| Cu11 | 22.02 | Cu25(b) | 57.35 | Cu40 | 98.53 | Cu57 | 146.41 | Cu75 | 193.34 |
| 26.05 | 68.56 | 118.01 | 175.73 | 233.20 | |||||
| Cu12 | 24.36 | Cu26(a) | 60.66 | Cu41 | 100.45 | Cu58 | 149.39 | Cu76 | 196.20 |
| 28.76 | 72.33 | 120.31 | 179.14 | 236.56 | |||||
| Cu12 | 24.38 | Cu26(b) | 59.68 | Cu42 | 104.06 | Cu59 | 150.69 | Cu77 | 199.21 |
| 28.78 | 71.64 | 124.59 | 180.85 | 240.04 | |||||
| Cu13 | 26.32 | Cu27 | 62.62 | Cu43(a) | 106.50 | Cu60 | 154.45 | Cu78 | 202.35 |
| 31.58 | 74.90 | 127.66 | 185.30 | 243.34 | |||||
| Cu14 | 29.19 | Cu28(a) | 65.83 | Cu43(b) | 104.87 | Cu61 | 157.05 | Cu79 | 204.59 |
| 34.88 | 78.41 | 127.66 | 188.65 | 246.44 | |||||
| Cu15 | 32.21 | Cu28(b) | 65.42 | Cu44 | 109.00 | Cu62 | 159.56 | Cu80 | 207.37 |
| 38.38 | 78.24 | 130.74 | 191.59 | 249.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, H.-Y.; Li, B.-X.; Ding, W.-F.; Zhu, Y.-H.; Yang, X.-X.; Li, C.-Y.; Ye, G.-X. Theoretical Study on the Aggregation of Copper Clusters on a Liquid Surface. Materials 2019, 12, 3877. https://doi.org/10.3390/ma12233877
Mao H-Y, Li B-X, Ding W-F, Zhu Y-H, Yang X-X, Li C-Y, Ye G-X. Theoretical Study on the Aggregation of Copper Clusters on a Liquid Surface. Materials. 2019; 12(23):3877. https://doi.org/10.3390/ma12233877
Chicago/Turabian StyleMao, Hong-Ying, Bao-Xing Li, Wang-Feng Ding, Yu-Hong Zhu, Xu-Xin Yang, Chao-Yang Li, and Gao-Xiang Ye. 2019. "Theoretical Study on the Aggregation of Copper Clusters on a Liquid Surface" Materials 12, no. 23: 3877. https://doi.org/10.3390/ma12233877