Effect of YAl2 Particles on the Corrosion Behavior of Mg–Li Matrix Composite in NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
2.2. Immersion Test
2.3. Electrochemical Measurements
2.4. Microstructure Characterization
3. Results and Discussion
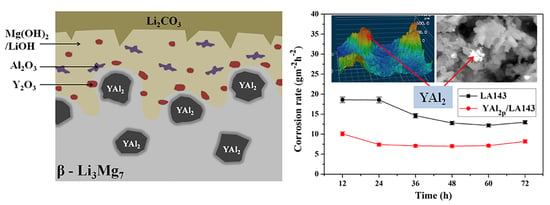
3.1. Immersion Test
3.2. Open Circuit Potential and Potentiodynamic Polarization Measurements
3.3. EIS Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Y.-N.; Wu, H.-Y.; Zhou, G.-Z.; Chiu, C.-H.; Lee, S. Mechanical and anisotropic behaviors of Mg–Li–Zn alloy thin sheets. Mater. Des. 2008, 29, 2061–2065. [Google Scholar] [CrossRef]
- Song, G.S.; Staiger, M.; Kral, M. Some new characteristics of the strengthening phase in β-phase magnesium–lithium alloys containing aluminum and beryllium. Mater. Sci. Eng. A 2004, 371, 371–376. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Li, Y.; Wang, H.; Liu, J.; Liaw, P.K.; Bei, H.; Zhang, Z. Improvement of mechanical behaviors of a superlight Mg-Li base alloy by duplex phases and fine precipitates. J. Alloys Compd. 2018, 735, 2625–2633. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Sun, L.; Zheng, Y.-F.; Cui, H.-Z.; Han, E.-H. Corrosion and characterisation of dual phase Mg–Li–Ca alloy in Hank’s solution: The influence of microstructural features. Corros. Sci. 2014, 79, 69–82. [Google Scholar] [CrossRef]
- Wu, R.-Z.; Yan, Y.-D.; Wang, G.-X.; Murr, L.E.; Han, W.; Zhang, Z.-W.; Zhang, M.-I. Recent progress in magnesium–lithium alloys. Int. Mater. Rev. 2015, 60, 65–100. [Google Scholar] [CrossRef]
- Pan, F.; Yang, M.; Chen, X. Technology, A review on casting magnesium alloys: modification of commercial alloys and development of new alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, G.; Wang, S.; Liu, Y.; Luo, H.; Technology, C. A chrome-free conversion coating for magnesium–lithium alloy by a phosphate–permanganate solution. Surf. Coat. Technol. 2008, 202, 1825–1830. [Google Scholar] [CrossRef]
- Song, G.; StJohn, D. Corrosion behaviour of magnesium in ethylene glycol. Corros. Sci. 2004, 46, 1381–1399. [Google Scholar] [CrossRef]
- Ballerini, G.; Bardi, U.; Bignucolo, R.; Ceraolo, G. About some corrosion mechanisms of AZ91D magnesium alloy. Corros. Sci. 2005, 47, 2173–2184. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hayashi, N.; Izumi, S.; Kawamura, Y. Corrosion behavior of rapidly solidified Mg–Zn–rare earth element alloys in NaCl solution. Corros. Sci. 2007, 49, 255–262. [Google Scholar] [CrossRef]
- Wang, S.; Wu, G.; Li, R.; Luo, G.; Huang, Z. Microstructures and mechanical properties of 5 wt.% Al2Yp/Mg–Li composite. Mater. Lett. 2006, 60, 1863–1865. [Google Scholar] [CrossRef]
- Tiwari, S.; Balasubramaniam, R.; Gupta, M. Corrosion behavior of SiC reinforced magnesium composites. Corros. Sci. 2007, 49, 711–725. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, S.; Merino, M.C.; Barroso, I.; Mohedano, M.; Arrabal, R.; Viejo, F. Corrosion behaviour of silicon–carbide-particle reinforced AZ92 magnesium alloy. Corros. Sci. 2009, 51, 841–849. [Google Scholar] [CrossRef]
- Hihara, L.H.; Latanision, R.M. Corrosion of metal matrix composites. Metall. Rev. 1994, 39, 245–264. [Google Scholar] [CrossRef]
- Shi, Z.; Song, G.; Atrens, A. The corrosion performance of anodised magnesium alloys. Corros. Sci. 2006, 48, 3531–3546. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Fekry, A.; Jibril, M.A.E.-B. Electrochemical behaviour of the Mg alloy AZ91D in borate solutions. Corros. Sci. 2011, 53, 1174–1185. [Google Scholar] [CrossRef]
- Verdier, S.; Van Der Laak, N.; Delalande, S.; Metson, J.; Dalard, F. The surface reactivity of a magnesium–aluminium alloy in acidic fluoride solutions studied by electrochemical techniques and XPS. Appl. Surf. Sci. 2004, 235, 513–524. [Google Scholar] [CrossRef]
- Hiromoto, S.; Shishido, T.; Yamamoto, A.; Maruyama, N.; Somekawa, H.; Mukai, T. Precipitation control of calcium phosphate on pure magnesium by anodization. Corros. Sci. 2008, 50, 2906–2913. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, X.; Zhang, M.; Gao, L.; Wu, R. Electrochemical characterization of the corrosion of a Mg–Li alloy. Mater. Lett. 2008, 62, 2177–2180. [Google Scholar] [CrossRef]
- Xiang, Q.; Jiang, B.; Zhang, Y.; Chen, X.; Song, J.; Xu, J.; Fang, L.; Pan, F. Effect of rolling-induced microstructure on corrosion behaviour of an as-extruded Mg-5Li-1Al alloy sheet. Corros. Sci. 2017, 119, 14–22. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Li, Y.; Yang, T. Effect of yttrium on the corrosion behavior of 09CrCuSb alloy in NaCl solution. Corros. Sci. 2014, 87, 211–217. [Google Scholar] [CrossRef]
- Jönsson, M.; Dan, P.; Thierry, D. Corrosion product formation during NaCl induced atmospheric corrosion of magnesium alloy AZ91D. Corros. Sci. 2007, 49, 1540–1558. [Google Scholar] [CrossRef]
- Xin, Y.; Huo, K.; Hu, T.; Tang, G.; Chu, P.K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Corros. Sci. 2008, 4, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, G.; Huang, Z.; Tao, Y. Compounds, Effects of particle/matrix interfaces on the mechanical properties for SiCp or YAl2p reinforced Mg–Li composites. J. Alloys Compd. 2014, 588, 1–6. [Google Scholar] [CrossRef]
- Luo, T.; Yang, Y.J.M. Design, Corrosion properties and corrosion evolution of as-cast AZ91 alloy with rare earth yttrium. Mater. Des. 2011, 32, 5043–5048. [Google Scholar] [CrossRef]
- Nouri, M.; Sun, X.; Li, D. Beneficial effects of yttrium on the performance of Mg–3% Al alloy during wear, corrosion and corrosive wear. Tribol. Int. 2013, 67, 154–163. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Deng, X.; Hongwei, L.; Yongjun, L.; Minglong, M.; Ning, L.; Wang, Y. Corrosion behavior of Mg–Y alloy in NaCl aqueous solution. Prog. Nat. Sci. Mater. Int. 2012, 22, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, T.; Andersson, A.M.; Bishop, A.G.; Gejke, C.; Gustafsson, T.; Thomas, J. Surface Analysis of LiMn2 O 4 Electrodes in Carbonate-Based Electrolytes. J. Electrochem. Soc. 2002, 149, A69–A78. [Google Scholar] [CrossRef]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kendig, M.; Tsai, S. Evaluation of corrosion behavior of coated metals with AC impedance measurements. Corrosion 1982, 38, 478–485. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Li, K.; Yan, J.; Li, Y.; Feng, J. Effect of yttrium on the corrosion behaviour of 09CrCuSb alloy in concentrated sulphuric acid. Corros. Sci. 2013, 69, 369–375. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardaş, G.; Culha, M.; Yazıcı, B.; Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Özcan, M.; Dehri, I.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]
- Özcan, M.; Karadağ, F.; Dehri, I.J.C.; Physicochemical, S.A.; Aspects, E. Investigation of adsorption characteristics of methionine at mild steel/sulfuric acid interface: an experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 55–61. [Google Scholar] [CrossRef]
- Oguzie, E.; Li, Y.; Wang, F. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Behpour, M.; Ghoreishi, S.; Soltani, N.; Salavati-Niasari, M. The inhibitive effect of some bis-N, S-bidentate Schiff bases on corrosion behaviour of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 1073–1082. [Google Scholar] [CrossRef]
- Umoren, S.; Li, Y.; Wang, F. Synergistic effect of iodide ion and polyacrylic acid on corrosion inhibition of iron in H2SO4 investigated by electrochemical techniques. Corros. Sci. 2010, 52, 2422–2429. [Google Scholar] [CrossRef]











| Element | Weight Percentage (wt.%) | ||
|---|---|---|---|
| Spot 1 | Spot 2 | Spot 3 | |
| C | 0.70 | 0.60 | 0.72 |
| O | 7.25 | 5.02 | 5.10 |
| Mg | 16.70 | 30.99 | 44.85 |
| Al | 15.76 | 7.24 | 7.44 |
| Cl | 0 | 1.34 | 0.87 |
| Cr | 31.43 | 46.85 | 33.58 |
| Y | 28.16 | 7.96 | 7.43 |
| Samples | Rcl/Ω·cm−2 | Ccl/F·cm−2 | Rct/Ω·cm−2 | Qdl/F·cm−2 |
|---|---|---|---|---|
| LA143 alloy | 27.59 ± 0.01 | (4.139 ± 0.001) × 10−5 | 13.56 ± 0.01 | (2.083 ± 0.008) × 10−4 |
| YAl2p/LA143 composite | 61.35 ± 0.02 | (2.445 ± 0.001) × 10−5 | 17.02 ± 0.02 | (1.350 ± 0.01) × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Bao, C.; Wu, G.; Jian, Y.; Zhang, L. Effect of YAl2 Particles on the Corrosion Behavior of Mg–Li Matrix Composite in NaCl Solution. Materials 2019, 12, 549. https://doi.org/10.3390/ma12030549
Chen Z, Bao C, Wu G, Jian Y, Zhang L. Effect of YAl2 Particles on the Corrosion Behavior of Mg–Li Matrix Composite in NaCl Solution. Materials. 2019; 12(3):549. https://doi.org/10.3390/ma12030549
Chicago/Turabian StyleChen, Zihan, Chonggao Bao, Guoqing Wu, Yongxin Jian, and Li Zhang. 2019. "Effect of YAl2 Particles on the Corrosion Behavior of Mg–Li Matrix Composite in NaCl Solution" Materials 12, no. 3: 549. https://doi.org/10.3390/ma12030549