Influence of Physical Activity during Pregnancy on Maternal Hypertensive Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
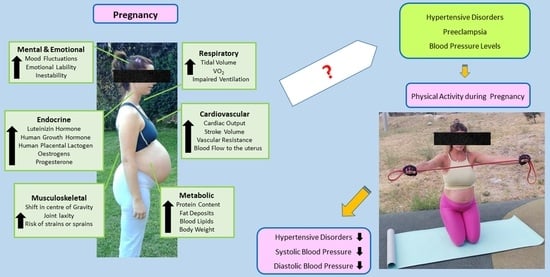
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Intervention
2.3. Comparison
2.4. Outcomes
2.5. Study Design and Selection Process
2.6. Statistical Analysis, Quality of Evidence Assessment, Risk of Bias, and Publication Bias
3. Results
3.1. Study Selection
3.2. Risk of Bias Assessment
3.3. Effect of PA on the Occurrence of Hypertensive Disorders
3.4. Effect of PA during Pregnancy on Diagnosed Preeclampsia
3.5. Effect of PA during Pregnancy on Systolic Blood Pressure
3.6. Effect of PA during Pregnancy on Diastolic Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrawus, M.; Sharvit, L.; Atzmon, G. Epigenetics and Pregnancy: Conditional Snapshot or Rolling Event. Int. J. Mol. Sci. 2022, 23, 12698. [Google Scholar] [CrossRef] [PubMed]
- Zuccarello, D.; Sorrentino, U.; Brasson, V.; Marin, L.; Piccolo, C.; Capalbo, A.; Andrisani, A.; Cassina, M. Epigenetics of pregnancy: Looking beyond the DNA code. J. Assist. Reprod. Genet. 2022, 39, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Best, J.D.; Carey, N. The Epigenetics of Normal Pregnancy. Obstet. Med. 2013, 6, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Cameron, N.A.; Lindley, K.J. Pregnancy as an Early Cardiovascular Moment: Peripartum Cardiovascular Health. Circ. Res. 2023, 132, 1584–1606. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Lin, H.C.; Tsai, M.L.; Chang, Y.T.; Chang, Y.C. Maternal hypertensive pregnancy disorders increase childhood intellectual disability hazards independently from preterm birth and small for gestational age. Early Hum. Dev. 2023, 185, 105856. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin, No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25.
- Folk, D.M. Hypertensive Disorders of Pregnancy: Overview and Current Recommendations. J. Midwifery Women’s Health 2018, 63, 289–300. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef]
- Atkinson, S.A.; Maran, A.; Dempsey, K.; Perreault, M.; Vanniyasingam, T.; Phillips, S.M.; Hutton, E.K.; Mottola, M.F.; Wahoush, O.; Xie, F.; et al. Be Healthy in Pregnancy (BHIP): A Randomized Controlled Trial of Nutrition and Exercise Intervention from Early Pregnancy to Achieve Recommended Gestational Weight Gain. Nutrients 2022, 14, 810. [Google Scholar] [CrossRef] [PubMed]
- Babbar, S.; Hill, J.B.; Williams, K.B.; Pinon, M.; Chauhan, S.P.; Maulik, D. Acute feTal behavioral Response to prenatal Yoga: A single, blinded, randomized controlled trial (TRY yoga). Am. J. Obstet. Gynecol. 2016, 214, 399.e1–399.e8. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Montejo, R.; Luaces, M.; Zakynthinaki, M. Exercise during pregnancy improves maternal health perception: A randomized controlled trial. Am. J. Obstet. Gynecol. 2011, 204, 402.e1–402.e7. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Cordero, Y.; Coteron, J.; Luaces, M.; Montejo, R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: A randomised controlled trial. Br. J. Sports Med. 2012, 46, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Lopez, C.; Montejo, R.; Coteron, J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: Results of a randomized controlled trial. J. Matern. Fetal Neonatal Med. 2012, 25, 2372–2376. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Perales, M.; Bacchi, M.; Coteron, J.; Refoyo, I. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am. J. Health Promot. 2014, 29, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; Lopez, C.; Coterón, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016, 214, 649.e1–649.e8. [Google Scholar] [CrossRef] [PubMed]
- Bhartia, N.; Jain, S.; Shankar, N.; Rajaram, S.; Gupta, M. Effects of antenatal yoga on maternal stress and clinical outcomes in north indian women: A randomised controlled trial. J. Indian. Acad. Clin. Med. 2019, 20, 11. [Google Scholar]
- Da Silva, S.G.; Hallal, P.C.; Domingues, M.R.; Bertoldi, A.D.; da Silveira, M.F.; Bassani, D.; da Silva, I.C.M.; da Silva, B.G.C.; Coll, C.V.N.; Evenson, K. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 175. [Google Scholar] [CrossRef]
- De Oliveria Melo, A.S.; Silva, J.L.; Tavares, J.S.; Barros, V.O.; Leite, D.F.; Amorim, M.M. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstet. Gynecol. 2012, 120 Pt 1, 302–310. [Google Scholar] [CrossRef]
- Fernández-Buhigas, I.; Brik, M.; Martin-Arias, A.; Vargas-Terrones, M.; Varillas, D.; Barakat, R.; Santacruz, B. Maternal physiological changes at rest induced by exercise during pregnancy: A randomized controlled trial. Physiol. Behav. 2020, 220, 112863. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, K.J.; Ong, M.J.; Crisp, N.A.; Fournier, P.A.; Wallman, K.E.; Grove, R.J.; Doherty, D.A.; Newnham, J.P. Regular Exercise to Prevent the Recurrence of Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 128, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Haakstad, L.A.; Bø, K. The marathon of labour—Does regular exercise training influence course of labour and mode of delivery?: Secondary analysis from a randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Karthiga, K.; Pal, G.K.; Dasari, P.; Nanda, N.; Velkumary, S.; Chinnakali, P.; Renugasundari, M.; Harichandrakumar, K.T. Effects of yoga on cardiometabolic risks and fetomaternal outcomes are associated with serum nitric oxide in gestational hypertension: A randomized control trial. Sci. Rep. 2022, 12, 11732. [Google Scholar] [CrossRef] [PubMed]
- Khoram, S.; Loripoor, M.; Pirhadi, M.; Beigi, M. The effect of walking on pregnancy blood pressure disorders in women susceptible to pregnancy hypertension: A randomized clinical trial. J. Educ. Health Promot. 2019, 8, 95. [Google Scholar] [PubMed]
- Koivusalo, S.B.; Rönö, K.; Klemetti, M.M.; Roine, R.P.; Lindström, J.; Erkkola, M.; Kaaja, R.J.; Pöyhönen-Alho, M.; Tiitinen, A.; Huvinen, E. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL): A Randomized Controlled Trial. Diabetes Care 2016, 39, 24–30. [Google Scholar] [CrossRef]
- Kong, K.L.; Campbell, C.G.; Foster, R.C.; Peterson, A.D.; Lanningham-Foster, L. A pilot walking program promotes moderate-intensity physical activity during pregnancy. Med. Sci. Sports Exerc. 2014, 46, 462–471. [Google Scholar] [CrossRef]
- Luoto, R.; Kinnunen, T.I.; Aittasalo, M.; Kolu, P.; Raitanen, J.; Ojala, K.; Mansikkamäki, K.; Lamberg, S.; Vasankari, T.; Komulainen, T.; et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: A cluster-randomized controlled trial. PLoS Med. 2011, 8, e1001036. [Google Scholar] [CrossRef]
- Murphy, S.E.; Johnston, C.A.; Strom, C.; Isly, C.; Haven, K.; Newton, E.; McDonald, S.; May, L.E. Influence of exercise type on maternal blood pressure adaptation throughout pregnancy. AJOG Glob. Rep. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Perales, M.; Santos-Lozano, A.; Sanchis-Gomar, F.; Luaces, M.; Pareja-Galeano, H.; Garatachea, N.; Barakat, R.; Lucía, A. Maternal Cardiac Adaptations to a Physical Exercise Program during Pregnancy. Med. Sci. Sports Exerc. 2016, 48, 896–906. [Google Scholar] [CrossRef]
- Petrov Fieril, K.; Glantz, A.; Fagevik Olsen, M. The efficacy of moderate-to-vigorous resistance exercise during pregnancy: A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2015, 94, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Price, B.B.; Amini, S.B.; Kappeler, K. Exercise in pregnancy: Effect on fitness and obstetric outcomes-a randomized trial. Med. Sci. Sports Exerc. 2012, 44, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, L.; Ruiz-Frutos, C.; Vázquez-Lara, J.M.; Ramírez-Rodrigo, J.; Villaverde-Gutiérrez, C.; Torres-Luque, G. Effectiveness of a physical activity programme based on the Pilates method in pregnancy and labour. Efectividad de un programa de actividad física mediante el método Pilates en el embarazo y en el proceso del parto. Enferm. Clin. 2017, 27, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Perales, M.; Pelaez, M.; Lopez, C.; Lucia, A.; Barakat, R. Supervised exercise-based intervention to prevent excessive gestational weight gain: A randomized controlled trial. Mayo Clin. Proc. 2013, 88, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Sagedal, L.R.; Øverby, N.C.; Bere, E.; Torstveit, M.K.; Lohne-Seiler, H.; Smastuen, M.; Hillesund, E.R.; Henriksen, T.; Vistad, I. Lifestyle intervention to limit gestational weight gain: The Norwegian Fit for Delivery randomised controlled trial. BJOG 2017, 124, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Silva-Jose, C.; Sánchez-Polán, M.; Diaz-Blanco, Á.; Coterón, J.; Barakat, R.; Refoyo, I. Effectiveness of a Virtual Exercise Program during COVID-19 Confinement on Blood Pressure Control in Healthy Pregnant Women. Front. Physiol. 2021, 12, 645136. [Google Scholar] [CrossRef]
- Skow, R.J.; Fraser, G.M.; Steinback, C.D.; Davenport, M.H. Prenatal Exercise and Cardiovascular Health (PEACH) Study: Impact on Muscle Sympathetic Nerve (Re)Activity. Med. Sci. Sports Exerc. 2021, 53, 1101–1113. [Google Scholar] [CrossRef]
- Stafne, S.N.; Salvesen, K.Å.; Romundstad, P.R.; Eggebø, T.M.; Carlsen, S.M.; Mørkved, S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet. Gynecol. 2012, 119, 29–36. [Google Scholar] [CrossRef]
- Stutzman, S.S.; Brown, C.A.; Hains, S.M.; Hains, S.M.J.; Godwin, M.; Smith, G.N.; Parlow, J.L.; Kisilevsky, B.S. The effects of exercise conditioning in normal and overweight pregnant women on blood pressure and heart rate variability. Biol. Res. Nurs. 2010, 12, 137–148. [Google Scholar] [CrossRef]
- Taniguchi, C.; Sato, C. Home-based walking during pregnancy affects mood and birth outcomes among sedentary women: A randomized controlled trial. Int. J. Nurs. Pract. 2016, 22, 420–426. [Google Scholar] [CrossRef]
- Tomić, V.; Sporiš, G.; Tomić, J.; Milanović, Z.; Zigmundovac-Klaić, D.; Pantelić, S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat. Med. J. 2013, 54, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ussher, M.; Lewis, S.; Aveyard, P.; Manyonda, I.; West, R.; Lewis, B.; Marcus, B.; Riaz, M.; Taylor, A.; Daley, A.; et al. Physical activity for smoking cessation in pregnancy: Randomised controlled trial. BMJ 2015, 350, h2145. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Lara, J.M.; Rodríguez Díaz, L.; Ramírez Rodrigo, J.; Villaverde Gutiérrez, C.; Torres Luque, G.; Gómez-Salgado, J. Calidad de vida relacionada con la salud en una población de gestantes sanas tras un programa de actividad física en el medio acuático (PAFMAE) [Quality of life related to health in a population of healthy pregnant women after a program of physical activity in the aquatic environment.]. Rev. Esp. Salud Publica 2017, 91, e201710042. [Google Scholar] [PubMed]
- Yeo, S. Prenatal stretching exercise and autonomic responses: Preliminary data and a model for reducing preeclampsia. J. Nurs. Scholarsh 2010, 42, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Ferreira González, I.; Urrútia, G.; Alonso-Coello, P. Systematic reviews and meta-analysis: Scientific rationale and interpretation. Rev. Esp. Cardiol. 2011, 64, 688–696. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.; Vist, G.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Physical Activity and Exercise during Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. Obstet. Gynecol. 2020, 135, e178–e188. [CrossRef]
- Teede, H.J.; Bailey, C.; Moran, L.J.; Bahri Khomami, M.; Enticott, J.; Ranasinha, S.; Rogozińska, E.; Skouteris, H.; Boyle, J.A.; Thangaratinam, S.; et al. Association of Antenatal Diet and Physical Activity-Based Interventions with Gestational Weight Gain and Pregnancy Outcomes: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2022, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.; Andrade, A.; Nunes, I. Physical Exercise in Pregnancy: Benefits, Risks and Prescription. J. Perinat. Med. 2022, 50, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Dipietro, L.; Evenson, K.R.; Bloodgood, B.; Sprow, K.; Troiano, R.P.; Piercy, K.L.; Vaux-Bjerke, A.; Powell, K.E. 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE*. Benefits of Physical Activity during Pregnancy and Postpartum: An Umbrella Review. Med. Sci. Sports Exerc. 2019, 51, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.K.; Matchkov, V.V. Hypertension and physical exercise: The role of oxidative stress. Medicina 2016, 52, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Skow, R.J.; King, E.C.; Steinback, C.D.; Davenport, M.H. Davenport; The influence of prenatal exercise and pre-eclampsia on maternal vascular function. Clin. Sci. 2017, 131, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Magro-Malosso, E.R.; Saccone, G.; Di Tommaso, M.; Roman, A.; Berghella, V. Exercise during pregnancy and risk of gestational hypertensive disorders: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2017, 96, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vizcaíno, V.; Sanabria-Martínez, G.; Fernández-Rodríguez, R.; Cavero-Redondo, I.; Pascual-Morena, C.; Álvarez-Bueno, C.; Martínez-Hortelano, J.A. Exercise during pregnancy for preventing gestational diabetes mellitus and hypertensive disorders: An umbrella review of randomised controlled trials and an updated meta-analysis. BJOG 2023, 130, 264–275. [Google Scholar] [CrossRef]
- Wolferz, R.; Fotoohi, M.; Spiess, S.; Ose, D.M.P.H.; Vukelic, B.; Fortenberry, K.T. Does Exercise during Pregnancy Decrease the Risk of Developing Hypertensive Disorders? Am. Fam. Physician 2022, 106, 87–88. [Google Scholar]
- Zhang, J.; Wang, H.P.; Wang, X.X. Effects of aerobic exercise performed during pregnancy on hypertension and gestational diabetes: A systematic review and meta-analysis. J. Sports Med. Phys. Fit. 2023, 63, 852–863. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo García, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef]
- Meher, S.; Duley, L. Exercise or other physical activity for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2006, 2006, CD005942. [Google Scholar] [CrossRef] [PubMed]






| Author | Year | Country | N | EG | CG | Intervention: Physical Exercise Program | Main Variables Analyzed | Secondary Variables Analyzed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq | Intensity | Duration of Program | Type of Exercise | Superv. Classes | Duration of Class | Adh. | ||||||||
| Atkinson [11] | 2022 | Canada | 241 | 119 | 122 | 3–4 | Mod | 22 w | Walking | No | 25–40 min | 80% | Gestational weight gain | Hypertension, type of delivery and birth weight |
| Babbar [12] | 2016 | USA | 46 | 23 | 23 | 3 | Mod | 8 w | Yoga | Yes | 60 min | 80% | Umbilical artery, type of delivery, birth weight | Gestational weight gain, hypertension |
| Barakat [13] | 2011 | Spain | 80 | 40 | 40 | 3 | Low–Mod | 28 w | Aerobic and light strength, PFMT | Yes | 35–45 min | 90% | Maternal health status, urinary incontinence | Gestational age, type of delivery, hypertension |
| Barakat [14] | 2012 | Spain | 83 | 40 | 43 | 3 | Low–Mod | 28 w | Land aerobic and aquatic activity | Yes | 35–45 min | - | Gestational weight gain and gestational diabetes | Type of delivery, birth weight, hypertension |
| Barakat [15] | 2012 | Spain | 290 | 138 | 152 | 3 | Mod | 28 w | Aerobic exercise | Yes | 40–45 min | - | Type of delivery | Gestational weight gain, birth weight, hypertension |
| Barakat [16] | 2014 | Spain | 200 | 107 | 93 | 3 | Low–Mod | 28 w | Aerobic exercise, PFMT | Yes | 55–60 min | 80% | Gestational weight gain, type of delivery, hypertension, gestational diabetes | Birth weight, head circumference |
| Barakat [17] | 2016 | Spain | 765 | 382 | 383 | 3 | Mod | 28 w | Aerobic, strength, and flexibility exercise | Yes | 50–55 min | 80% | Hypertension, macrosomia | Type of delivery, gestational weight gain, birth weight |
| Bhartia [18] | 2019 | India | 78 | 38 | 40 | 1 | Mod | 12 w | Yoga | Yes | 50 min | - | Maternal stress, type of delivery, birth weight | Hypertension |
| 2 | No | |||||||||||||
| da Silva [19] | 2017 | Brazil | 639 | 213 | 426 | 3 | Mod | 16 w | Aerobic, strength training | Yes | 60 min | 70% | Preterm birth, preeclampsia, hypertension | Gestational weight gain, birth weight |
| de Oliveria [20] | 2012 | Brazil | 111 | 54 | 57 | 3 | 60–80% Max HR | 25 w | Walking | Yes | 15–40 min | 85% | VO2max, birth weight and gestational age | Hypertension |
| Fernández-Buhigas [21] | 2020 | Spain | 92 | 41 | 51 | 3 | 50–60% Max HR | 28 w | Aerobic and light strength, PFMT | Yes | 60 min | 70% | Hypertension, metabolic, hepatic | Renal |
| Guelfi [22] | 2016 | Australia | 172 | 85 | 87 | 3 | Mod | 14 w | Home-based stationary cycling program | Yes | 20–60 min | - | Gestational diabetes | Type of delivery, birth weight, hypertension |
| Haakstad [23] | 2020 | Norway | 90 | 43 | 47 | 2 | Mod | 12 w | Aerobic, PFMT | Yes | 60 min | 80% | Duration of labor, type of delivery, episiotomy | - |
| 3 | No | 30 min | ||||||||||||
| Karthiga [24] | 2022 | India | 234 | 121 | 113 | 7 | Mod | 20 w | Yoga Five sessions of yoga techniques | No | 60 min | - | Gestational hypertension | Type of delivery, duration of labor, birth weight |
| Khoram [25] | 2019 | Iran | 74 | 38 | 36 | 4 | Mod | 20 w | Walking | No | 20–30 min | - | Gestational hypertension | Preeclampsia |
| Koivusalo [26] | 2016 | Finland | 293 | 155 | 138 | 5 | Mod | 22 w | Aerobic, dietary counseling | No | 30 min | - | Gestational diabetes | Hypertension |
| Kong [27] | 2014 | USA | 37 | 18 | 19 | 5 | Mod | 22 w | Walking | No | 30 min | - | BMI, gestational weight gain | Hypertension |
| Luoto [28] | 2011 | Finland | 399 | 219 | 180 | 7 | Mod | 27 w | Aerobic, dietary counseling | No | 30 min | - | Gestational diabetes, gestational age | Hypertension |
| Murphy [29] | 2022 | USA | 154 | 63 | 21 | 3 | Mod | 20 w | Aerobic | Yes | 50 min | - | Hypertension | Heart rate |
| 33 | Resistance | |||||||||||||
| 37 | Combination | |||||||||||||
| Perales [30] | 2016 | Spain | 241 | 120 | 121 | 3 | Light Mod | 28 w | Aerobic and strength exercises | Yes | 55–60 min | - | Gestational weight gain, depression, hypertension | Duration of labor, type of delivery, birth weight, |
| Petrov Fieril [31] | 2015 | Sweden | 72 | 38 | 34 | 2 | Mod | 12 w | Resistance training | Yes | 60 min | - | Birth weight, gestational age | Hypertension, miscarriage |
| Price [32] | 2012 | USA | 62 | 31 | 31 | 4 | Mod | 23 w | Aerobic exercise | Yes | 45–60 min | - | Birth weight | Duration of labor, type of delivery, hypertension |
| Rodriguez-Diaz [33] | 2017 | Spain | 100 | 50 | 50 | 2 | Mod | 8 w | Pilates | Yes | 40–45 min | 90% | Gestational weight gain, hypertension, strength, flexibility and spinal curvature | Type of delivery, episiotomy, analgesia, and birth weight |
| Ruiz [34] | 2013 | Spain | 962 | 481 | 481 | 3 | Light mod | 28 w | Aerobic and resistance exercises | Yes | 50–55 min | 97% | Gestational weight gain | Hypertension, birth weight, type of delivery |
| Sagedal [35] | 2017 | Norway | 591 | 296 | 295 | 2 | Mod | 24 w | Aerobic, strength training. Dietary counselling | Yes | 60 min | - | Gestational weight gain, birth weight | Hypertension, gestational age, perineal tear |
| Silva-Jose [36] | 2021 | Spain | 72 | 31 | 41 | 3 | Mod | 28 w | Aerobic, strength exercises, PFMT | Yes | 55–60 min | 80% | Hypertension | Gestational weight gain |
| Skow [37] | 2021 | Canada | 59 | 31 | 28 | 3–4 | 50–70% Max HR | 17 w | Aerobic exercises | No | 25–40 min | - | Muscle sympathetic nerve activity | Gestational hypertension |
| Stafne [38] | 2012 | Norway | 855 | 429 | 426 | 1 | Mod–High | 12 w | Aerobic, strength exercise | Yes | 60 min | 55% | Gestational diabetes | Hypertension |
| 2 | No | 45 min | ||||||||||||
| Stutzman [39] | 2010 | Canada | 22 | 11 | 11 | 5 | Low | 16 w | Walking | No | 30–50 min | Hypertension, heart rate variability | Baroreflex sensitivity | |
| Taniguchi [40] | 2016 | Japan | 118 | 60 | 58 | 3 | Mod | 6 + w | Brisk walking | Yes | 30 min | 80% | Duration of labor, type of delivery, birth weight | - |
| Tomic [41] | 2013 | Croatia | 334 | 166 | 168 | 3 | 60–75% Max HR | 28 w | Aerobic exercise | Yes | 50 min | 80% | Hypertension, birth weight, type of delivery | Gestational weight gain |
| Ussher [42] | 2015 | UK | 785 | 392 | 393 | 2 | Mod | 6 w | Walking | Yes | 30 min | 70% | Smoking cessation, hypertension | Type of delivery, birth weight |
| Vázquez Lara [43] | 2017 | Spain | 46 | 18 | 28 | 2 | Mod | 6 w | Aquatic activities | Yes | 45 min | 90% | Hypertension | Plasma volume |
| Yeo [44] | 2010 | USA | 124 | 60 | 64 | 5 | Mod | 18 w | Stretching exercise | No | - | 75% | Preeclampsia | Hypertension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, R.; Silva-Jose, C.; Zhang, D.; Sánchez-Polán, M.; Refoyo, I.; Montejo, R. Influence of Physical Activity during Pregnancy on Maternal Hypertensive Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2024, 14, 10. https://doi.org/10.3390/jpm14010010
Barakat R, Silva-Jose C, Zhang D, Sánchez-Polán M, Refoyo I, Montejo R. Influence of Physical Activity during Pregnancy on Maternal Hypertensive Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Personalized Medicine. 2024; 14(1):10. https://doi.org/10.3390/jpm14010010
Chicago/Turabian StyleBarakat, Rubén, Cristina Silva-Jose, Dingfeng Zhang, Miguel Sánchez-Polán, Ignacio Refoyo, and Rocío Montejo. 2024. "Influence of Physical Activity during Pregnancy on Maternal Hypertensive Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Personalized Medicine 14, no. 1: 10. https://doi.org/10.3390/jpm14010010