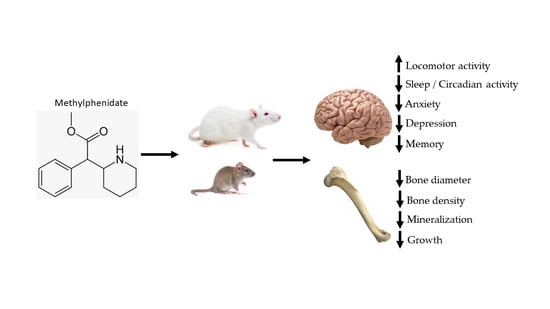
Behavioral, Neurochemical and Developmental Effects of Chronic Oral Methylphenidate: A Review
Abstract
:1. Introduction
1.1. History of Methylphenidate (MP)
1.2. MP Prescription and Use in Humans
1.3. MP Off-Label Use and Abuse
2. Clinical: Effects of Oral MP on Behavior and Medicine
3. Clinical: Effects of Oral MP on Brain Function and Neurochemistry
4. Preclinical Models of MP Treatment
4.1. Intraperitoneal Injection of MP
4.1.1. Effects of Injected MP on Behavior
4.1.2. Effects of Injected MP on Brain Function and Neurochemistry
| MP Exposure | Behavioral Effects | Model Used/References | Neurochemical Effects | Model Used/References |
|---|---|---|---|---|
| Chronic | Decreased hyperactive behavior Decreased self-administration and reinstatement of drug-seeking behavior Decreased drug sensitization and tolerance in exploratory and object recognition memory Impaired spatial and working memory results in decreased sensitivity to reward stimuli Increased locomotor activity compared to gavage administration Increased depressive and anxiety-like behavior | Naples high-excitability rats [58] Spotaneously hypertensive rats [59] Wistar rats [60] Wistar rats [57] Sprague-Dawley rats [51] | Induces oxidative damage, inflammatory changes, and neurodegeneration to the brain due to increased lipid peroxidation or mitochondrial superoxide DNA damage in striatal cells due to dopamine oxidation Enhanced pyramidal activity in adult rats Decreased synaptic transmission and neuronal excitability in juvenile rats Loss of astrocytes and neurons with increased levels of cytokines and neurotrophins in juvenile rats | Wistar rats [65,66] Sprague-Dawley rats [1] Wistar rats [60] |
| Acute | Decreased sensitivity to a given reward, Decreased habituation to a familiar environment and Increased depressive-like behavior Increased cross-sensitization suggests increased risk of future drug abuse Increased cocaine self-administration by rewarding effects and sensitivity of a given drug | Sprague-Dawley rats [61] Wistar rats [62] Sprague-Dawley rats [63] Sprague-Dawley rats [64] | Neuroprotective effects observed via the reduction in cell damage and decreased apoptosis in brain tissue | Sprague-Dawley rats [68] |
4.2. MP Oral Gavage
4.2.1. Effects of Gavage MP on Behavior
4.2.2. Effects of Gavage MP on Brain Function and Neurochemistry
| Duration of MP Exposure | Behavioral Effects | Model Used/References | Neurochemical Effects | Model Used/References |
|---|---|---|---|---|
| Chronic | Decreased animal stress Depressive-like behavior linked to decreases in hippocampal cell proliferation No evidence of changes in locomotor sensitization in adolescent rats | C57Bl/6J mice [70] Wistar rats [76] | Increased plasma corticosterone Increased dopamine levels in the brain Decreases hippocampal neurogenesis | C57Bl/6J mice [70] Sprague-Dawley rats [51] Wistar rats [76,80] |
| Acute | Increases animal stress Impairment of maternal behavior in female mice can produce pups with Increases anxiety-like behavior when they reach adulthood. Alleviates anxiety in Kv1.3 knockout mice | C57Bl/6J mice [70] Inbred BALB C mice [77] Super-Smeller, Kv1.3 Knockout mice [78] | Increases plasma corticosterone | C57Bl/6J mice [70] |
4.3. MP Oral Voluntary Drinking
4.3.1. Behavioral Effects of Chronic Oral MP Treatment
- i.
- Open Field Locomotor Activity
- ii.
- Sleep/Circadian Activity
- iii.
- Anxiety-Elevated Plus Maze (EPM)
- iv.
- Depression-Forced Swim Test (FST)
- v.
- Memory-Novel Object Recognition Memory
- vi.
- Cocaine-Conditioned Place Preference
| Route of MP Administration | Behavioral Effects | Model Used/References |
|---|---|---|
| Injected | Decreased hyperactive behavior Decreased self-administration and reinstatement of drug-seeking behavior Decreased drug sensitization and tolerance in exploratory and object recognition memory Impaired spatial and working memory results in Decreased sensitivity to reward stimuli Increased locomotor activity compared to gavage administration Increased depressive and anxiety-like behavior Decrease in body weight | Naples high-excitability rats [58] Spotaneously hypertensive rats [59] Wistar rats [60] Wistar rats [57] Sprague-Dawley rats [51] |
| Oral Gavage | Decreased animal stress Depressive-like behavior linked to decreases in hippocampal cell proliferation No evidence of changes in locomotor sensitization in adolescent rats | C57Bl/6J mice [70] Wistar rats [76] |
| Two-Bottle Paradigm | Increased locomotor activity Increased circadian activity No effect on sleep Decrease in anxiety in EPM Increase in latency to immobility during FST No effect on cocaine preference placement test Increase in food intake Decrease in body weight | Sprague-Dawley rats [81] Sprague-Dawley rats [90] Sprague-Dawley rats [14] Sprague-Dawley rats [85] |
4.3.2. Developmental Effects of Chronic Oral MP Exposure
- i.
- Food Intake
- ii.
- Body Weight
- iii.
- Skeletal Effects
4.3.3. Neurochemical Effects of Chronic MP
- i.
- DA Receptors
- ii.
- NMDA Glutamate Receptors
- iii.
- CB1 Cannabinoid Receptors
| Receptor | Brain Regions of Interest | Findings | Model Used/References |
|---|---|---|---|
| Dopamine |
|
| Sprague-Dawley rats [86] |
| Dopamine |
|
| Sprague-Dawley rats [40] |
| NMDA |
|
| Sprague-Dawley rats [101] |
| CB1 Cannabinoid |
|
| Sprague-Dawley rats [102] |
4.3.4. Effects of Chronic Oral MP on Brain Function and Structure
- i.
- MP Effects on Brain Function
- ii.
- MP Effects on Brain Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urban, K.R.; Waterhouse, B.D.; Gao, W.-J. Distinct Age-Dependent Effects of Methylphenidate on Developing and Adult Prefrontal Neurons. Biol. Psychiatry 2012, 72, 880–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, W.A.; Stockton, G.G. Methylphenidate Abuse and Psychiatric Side Effects. Prim. Care Companion J. Clin. Psychiatry 2000, 2, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kollins, S.H.; Rush, C.R.; Pazzaglia, P.J.; Ali, J.A. Comparison of acute behavioral effects of sustained-release and immediate-release methylphenidate. Exp. Clin. Psychopharmacol. 1998, 6, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Piper, B.J.; Ogden, C.L.; Simoyan, O.M.; Chung, D.Y.; Caggiano, J.F.; Nichols, S.D.; McCall, K.L. Trends in use of prescription stimulants in the United States and Territories, 2006 to 2016. PLoS ONE 2018, 13, e0206100. [Google Scholar] [CrossRef] [Green Version]
- McCabe, S.E.; Teter, C.J.; Boyd, C.J. Medical Use, Illicit Use, and Diversion of Abusable Prescription Drugs. J. Am. Coll. Health 2006, 54, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teter, C.J.; McCabe, S.E.; LaGrange, K.; Cranford, J.A.; Boyd, C.J. Illicit Use of Specific Prescription Stimulants Among College Students: Prevalence, Motives, and Routes of Administration. Pharmacother. J. Hum. Pharmacol. Drug. Ther. 2006, 26, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Kollins, S.H.; Wigal, T.L.; Newcorn, J.H.; Telang, F.W.; Fowler, J.S.; Logan, J.; Wong, C.T.; et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J. Neurosci. 2012, 32, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Frauger, E.; Amaslidou, D.; Spadari, M.; Allaria-Lapierre, V.; Braunstein, D.; Sciortino, V.; Thirion, X.; Djezzar, S.; Micallef, J. Patterns of Methylphenidate Use and Assessment of Its Abuse among the General Population and Individuals with Drug Dependence. Eur. Addict. Res. 2016, 22, 119–126. [Google Scholar] [CrossRef]
- Parasrampuria, D.A.; Schoedel, K.A.; Schuller, R.; Silber, S.A.; Ciccone, P.E.; Gu, J.; Sellers, E.M. Do formulation differences alter abuse liability of methylphenidate?—A placebo-controlled, randomized, double-blind, crossover study in recreational drug users. J. Clin. Psychopharmacol. 2007, 27, 459–467. [Google Scholar] [CrossRef]
- Roehrs, T.; Papineau, K.; Rosenthal, L.; Roth, T. Sleepiness and the Reinforcing and Subjective Effects of Methylphenidate. Exp. Clin. Psychopharmacol. 1999, 7, 145–150. [Google Scholar] [CrossRef]
- Schrantee, A.; Tamminga, H.G.; Bouziane, C.; Bottelier, M.A.; Bron, E.E.; Mutsaerts, H.J.M.; Zwinderman, A.H.; Groote, I.R.; Rombouts, S.A.; Lindauer, R.J.; et al. Age-Dependent Effects of Methylphenidate on the Human Dopaminergic System in Young vs. Adult Patients with Attention-Deficit/Hyperactivity Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Kola, V.P. 2638—Successful treatment of narcolepsy with methylphenidate (concerta). Eur. Psychiatry 2013, 28, 1. [Google Scholar] [CrossRef]
- Francisco, G.E.; Ivanhoe, C.B. Successful treatment of post-traumatic narcolepsy with methylphenidate: A case report. Am. J. Phys. Med. Rehabil. 1996, 75, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Robison, L.S.; Steier, J.; Hwang, Y.F.; Cooper, T.; Swanson, J.M.; Komatsu, D.E.; Hadjiargyrou, M.; Volkow, N.D. A pharmacokinetic model of oral methylphenidate in the rat and effects on behavior. Pharmacol. Biochem. Behav. 2015, 131, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbert, B.; Cohen, J.; Simon, N. Intravenous abuse of methylphenidate. J. Clin. Psychopharmacol. 2013, 33, 720–721. [Google Scholar] [CrossRef]
- Acosta, D.L.; Fair, C.N.; Gonzalez, C.M.; Iglesias, M.; Maldonado, N.; Schenkman, N.; Valle, S.M.; Velez, J.L.; Mejia, L. Nonmedical use of d-Amphetamines and Methylphenidate in Medical Students. P. R. Health Sci. J. 2019, 38, 185–188. [Google Scholar] [PubMed]
- Hadar, Y.; Hocherman, S.; Lamm, O.; Tirosh, E. The Visuo-Motor Attention Test in Boys with Attention Deficit Hyperactivity Disorder (ADHD): Methylphenidate-Placebo Randomized Controlled Trial. Child. Psychiatry Hum. Dev. 2021, 52, 96–103. [Google Scholar] [CrossRef]
- Leitner, Y.; Barak, R.; Giladi, N.; Peretz, C.; Eshel, R.; Gruendlinger, L.; Hausdorff, J.M. Gait in attention deficit hyperactivity disorder: Effects of methylphenidate and dual tasking. J. Neurol. 2007, 254, 1330–1338. [Google Scholar] [CrossRef]
- Flapper, B.C.; Houwen, S.; Schoemaker, M.M. Fine motor skills and effects of methylphenidate in children with attention-deficit-hyperactivity disorder and developmental coordination disorder. Dev. Med. Child. Neurol. 2006, 48, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Al-Adawi, S.; Burke, D.T.; Dorvlo, A.S.S. The effect of methylphenidate on the sleep-wake cycle of brain-injured patients undergoing rehabilitation. Sleep. Med. 2006, 7, 287–291. [Google Scholar] [CrossRef]
- Rhodes, S.M.; Coghill, D.R.; Matthews, K. Acute neuropsychological effects of methylphenidate in stimulant drug-naïve boys with ADHD II--broader executive and non-executive domains. J. Child. Psychol. Psychiatry 2006, 47, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Storebø, O.J.; Krogh, H.B.; Ramstad, E.; Moreira-Maia, C.R.; Holmskov, M.; Skoog, M.; Nilausen, T.D.; Magnusson, F.L.; Zwi, M.; Gillies, D.; et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015, 351, h5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodey, C. Effectiveness and Tolerability of Methylphenidate in Children and Adolescents with Attention Deficit Hyperactivity Disorder. Clin. Med. Insights: Ther. 2011, 2011, 353. [Google Scholar] [CrossRef] [Green Version]
- Molina-Carballo, A.; Naranjo-Gómez, A.; Uberos, J.; Justicia-Martínez, F.; Ruiz-Ramos, M.-J.; Cubero-Millán, I.; Contreras-Chova, F.; Augustin-Morales, M.-d.-C.; Khaldy-Belkadi, H.; Muñoz-Hoyos, A. Methylphenidate effects on blood serotonin and melatonin levels may help to synchronise biological rhythms in children with ADHD. J. Psychiatr. Res. 2012, 47, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kling, J. Methylphenidate formulation helps students with ADHD. Clin. Psychiatry News 2018, 46, 3. [Google Scholar]
- Rosenau, P.T.; Openneer TJ, C.; Matthijssen, A.-F.M.; van de Loo-Neus GH, H.; Buitelaar, J.K.; van den Hoofdakker, B.J.; Hoekstra, P.J.; Dietrich, A. Effects of methylphenidate on executive functioning in children and adolescents with ADHD after long-term use: A randomized, placebo-controlled discontinuation study. J. Child. Psychol. Psychiatry 2021, 62, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carbonell, L.; Mignot, E.; Leschziner, G.; Dauvilliers, Y. Understanding and approaching excessive daytime sleepiness. Lancet 2022, 400, 1033–1046. [Google Scholar] [CrossRef]
- Van Dijk, G.P.; Jansen, K.; Lagae, L.; Buyse, G. PP5.6—2044 Methylphenidate in children with narcolepsy/cataplexy. Eur. J. Paediatr. Neurol. 2013, 17, S43. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Telang, F.; Logan, J.; Wong, C.; Ma, J.; Pradhan, K.; Benveniste, H.; Swanson, J.M. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS ONE 2008, 3, e2017. [Google Scholar] [CrossRef] [Green Version]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef]
- Urban, K.R.; Gao, W.-J. Methylphenidate and the juvenile brain: Enhancement of attention at the expense of cortical plasticity? Med. Hypotheses 2013, 81, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, S. Methylphenidate works by increasing dopamine levels. BMJ 2001, 322, 259. [Google Scholar] [CrossRef] [Green Version]
- Kuczenski, R.; Segal, D.S. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J. Neurochem. 1997, 68, 2032–2037. [Google Scholar] [CrossRef]
- Daniali, S.; Madjd, Z.; Shahbazi, A.; Niknazar, S.; Shahbazzadeh, D. Chronic Ritalin administration during adulthood increases serotonin pool in rat medial frontal cortex. Iran. Biomed. J. 2013, 17, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Hofmans, L.; Papadopetraki, D.; van den Bosch, R.; Määttä, J.I.; Froböse, M.I.; Zandbelt, B.B.; Westbrook, A.; Verkes, R.J.; Cools, R. Methylphenidate boosts choices of mental labor over leisure depending on striatal dopamine synthesis capacity. Neuropsychopharmacol. 2020, 45, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Manza, P.; Shokri-Kojori, E.; Wiers, C.E.; Kroll, D.; Feldman, D.; McPherson, K.; Biesecker, E.; Dennis, E.; Johnson, A.; Kelleher, A.; et al. Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol. Psychiatry 2021, 27, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Tebartz van Elst, L.; Maier, S.; Klöppel, S.; Graf, E.; Killius, C.; Rump, M.; Sobanski, E.; Ebert, D.; Berger, M.; Warnke, A.; et al. The effect of methylphenidate intake on brain structure in adults with ADHD in a placebo-controlled randomized trial. J. Psychiatry Neurosci. 2016, 41, 422–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweren, L.J.; de Zeeuw, P.; Durston, S. MR imaging of the effects of methylphenidate on brain structure and function in attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2013, 23, 1151–1164. [Google Scholar] [CrossRef]
- Cepeda, C.; Levine, M.S. Where do you think you are going? The NMDA-D1 receptor trap. Sci. STKE 2006, 2006, pe20. [Google Scholar] [CrossRef]
- Thanos, P.K.; Michaelides, M.; Benveniste, H.; Wang, G.J.; Volkow, N.D. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharm. Biochem. Behav. 2007, 87, 426–433. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D.; Wang, G.J.; Wang, R.; Telang, F.; Caparelli, E.C.; Wong, C.; Jayne, M.; Fowler, J.S. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. NeuroImage 2011, 54, 3101–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, K.R.; Li, Y.C.; Gao, W.J. Treatment with a clinically-relevant dose of methylphenidate alters NMDA receptor composition and synaptic plasticity in the juvenile rat prefrontal cortex. Neurobiol. Learn. Mem. 2013, 101, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Gatley, S.J.; Logan, J.; Ding, Y.S.; Hitzemann, R.; Pappas, N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am. J. Psychiatry 1998, 155, 1325–1331. [Google Scholar] [CrossRef] [Green Version]
- Konrad, K.; Neufang, S.; Fink, G.R.; Herpertz-Dahlmann, B. Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: Results from a longitudinal functional MRI study. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.B.; Lee, D.O.; Hanford, R.B.; Tagamets, M.A.; Hoffman, J.M.; Grafton, S.T.; Kilts, C.D. A positron emission tomography study of methylphenidate in adults with ADHD: Alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology 2003, 28, 967–973. [Google Scholar] [CrossRef]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Ellenbroek, B.; Youn, J. Roden? models in neuroscience research: Is it a rat race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuczenski, R.; Segal, D.S. Stimulant Actions in Rodents: Implications for Attention-Deficit/Hyperactivity Disorder Treatment and Potential Substance Abuse. Biol. Psychiatry 2005, 57, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Gerasimov, M.R.; Franceschi, M.; Volkow, N.D.; Gifford, A.; Gatley, S.J.; Marsteller, D.; Molina, P.E.; Dewey, S.L. Comparison between Intraperitoneal and Oral Methylphenidate Administration: A Microdialysis and Locomotor Activity Study. J. Pharmacol. Exp. Ther. 2000, 295, 51. [Google Scholar]
- Bouchatta, O.; Manouze, H.; Bouali-Benazzouz, R.; Kerekes, N.; Ba-M’hamed, S.; Fossat, P.; Landry, M.; Bennis, M. Neonatal 6-OHDA lesion model in mouse induces Attention-Deficit/Hyperactivity Disorder (ADHD)-like behaviour. Sci. Rep. 2018, 8, 15349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaytan, O.; Ghelani, D.; Martin, S.; Swann, A.; Dafny, N. Dose response characteristics of methylphenidate on different indices of rats’ locomotor activity at the beginning of the dark cycle. Brain Res. 1996, 727, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.; Kanters, D.; Geers, F.; Buitelaar, J.K.; Kozicz, T.; Glennon, J.C. Methylphenidate Dose-Dependently Affects Aggression and Improves Fear Extinction and Anxiety in BALB/cJ Mice. Front. Psychiatry 2019, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Urban, K.R.; Gao, W.-J. Evolution of the Study of Methylphenidate and Its Actions on the Adult Versus Juvenile Brain. J. Atten. Disord. 2015, 19, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitonea? Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Scherer, E.B.S.; da Cunha, M.J.; Matté, C.; Schmitz, F.; Netto, C.A.; Wyse, A.T.S. Methylphenidate affects memory, brain-derived neurotrophic factor immunocontent and brain acetylcholinesterase activity in the rat. Neurobiol. Learn. Mem. 2010, 94, 247–253. [Google Scholar] [CrossRef]
- Ruocco, L.A.; Gironi Carnevale, U.A.; Treno, C.; Sadile, A.G.; Melisi, D.; Arra, C.; Ibba, M.; Schirru, C.; Carboni, E. Prepuberal subchronic methylphenidate and atomoxetine induce different long-term effects on adult behaviour and forebrain dopamine, norepinephrine and serotonin in Naples High-Excitability rats. Behav. Brain Res. 2010, 210, 99–106. [Google Scholar] [CrossRef] [PubMed]
- dela Peña, I.; Yoon, S.Y.; Lee, J.C.; dela Peña, J.B.; Sohn, A.R.; Ryu, J.H.; Shin, C.Y.; Cheong, J.H. Methylphenidate treatment in the spontaneously hypertensive rat: Influence on methylphenidate self-administration and reinstatement in comparison with Wistar rats. Psychopharmacology 2012, 221, 217–226. [Google Scholar] [CrossRef]
- Schmitz, F.; Pierozan, P.; Rodrigues, A.F.; Biasibetti, H.; Grunevald, M.; Pettenuzzo, L.F.; Scaini, G.; Streck, E.L.; Netto, C.A.; Wyse, A.T.S. Methylphenidate Causes Behavioral Impairments and Neuron and Astrocyte Loss in the Hippocampus of Juvenile Rats. Mol. Neurobiol. 2017, 54, 4201–4216. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Mague, S.D.; Andersen, S.L. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol. Psychiatry 2003, 54, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Valvassori, S.S.; Frey, B.N.; Martins, M.R.; Réus, G.Z.; Schimidtz, F.; Inácio, C.G.; Kapczinski, F.; Quevedo, J. Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav. Pharmacol. 2007, 18, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.A.; Baella, S.A.; Farley, C.M.; Herbert, M.S.; Horn, L.R.; Campbell, R.H.; Zavala, A.R. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology 2011, 213, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Brandon, C.L.; Marinelli, M.; Baker, L.K.; White, F.J. Enhanced Reactivity and Vulnerability to Cocaine Following Methylphenidate Treatment in Adolescent Rats. Neuropsychopharmacology 2001, 25, 651–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, K.M.; Inácio, C.G.; Valvassori, S.S.; Réus, G.Z.; Boeck, C.R.; Dal-Pizzol, F.; Quevedo, J. Superoxide production after acute and chronic treatment with methylphenidate in young and adult rats. Neurosci. Lett. 2009, 465, 95–98. [Google Scholar] [CrossRef]
- Martins, M.R.; Reinke, A.; Petronilho, F.C.; Gomes, K.M.; Dal-Pizzol, F.; Quevedo, J. Methylphenidate treatment induces oxidative stress in young rat brain. Brain Res. 2006, 1078, 189–197. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Frey, B.N.; Valvassori, S.S.; Zanotto, C.; Gomes, K.M.; Comim, C.M.; Cassini, C.; Stertz, L.; Ribeiro, L.C.; Quevedo, J.; et al. DNA damage in rats after treatment with methylphenidate. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1282–1288. [Google Scholar] [CrossRef]
- Li, P.; Huang, Y.; Yang, Y.; Huang, X. Methylphenidate exerts neuroprotective effects through the AMPK signaling pathway. Hum. Exp. Toxicol. 2021, 40, 1422–1433. [Google Scholar] [CrossRef]
- Witt, K.L.; Malarkey, D.E.; Hobbs, C.A.; Davis, J.P.; Kissling, G.E.; Caspary, W.; Travlos, G.; Recio, L. No increases in biomarkers of genetic damage or pathological changes in heart and brain tissues in male rats administered methylphenidate hydrochloride (Ritalin) for 28 days. Environ. Mol. Mutagen. 2010, 51, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Hoggatt, A.F.; Hoggatt, J.; Honerlaw, M.; Pelus, L.M. A spoonful of sugar helps the medicine go down: A novel technique to improve oral gavage in mice. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 329–334. [Google Scholar]
- Zhang-James, Y.; Lloyd, D.R.; James, M.L.; Yang, L.; Richards, J.B.; Faraone, S.V. Oral Methylphenidate Treatment of an Adolescent ADHD Rat Model Does Not Alter Cocaine-Conditioned Place Preference during Adulthood: A Negative Report. J. Psychiatry Brain Sci. 2019, 4, e190021. [Google Scholar] [CrossRef] [Green Version]
- Heal, D.J.; Pierce, D.M. Methylphenidate and its isomers: Their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs 2006, 20, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.; Stirling, D.; Thomas, S.; Hoberman, A.; Kiorpes, A.; Khetani, V. A 90-day oral gavage toxicity study of d-methylphenidate and d,l-methylphenidate in Sprague–Dawley rats. Toxicology 2002, 179, 183–196. [Google Scholar] [CrossRef]
- Bakhtiar, R.; Tse, F.L.S. Toxicokinetic assessment of methylphenidate (Ritalin®) in a 13-week oral (gavage) toxicity study in rats using an enantiomeric liquid chromatography/tandem mass spectrometry assay. Rapid Commun. Mass. Spectrom. 2003, 17, 2160–2162. [Google Scholar] [CrossRef]
- Van der Marel, K.; Klomp, A.; Meerhoff, G.F.; Schipper, P.; Lucassen, P.J.; Homberg, J.R.; Dijkhuizen, R.M.; Reneman, L. Long-Term Oral Methylphenidate Treatment in Adolescent and Adult Rats: Differential Effects on Brain Morphology and Function. Neuropsychopharmacology 2014, 39, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Van der Marel, K.; Bouet, V.; Meerhoff, G.F.; Freret, T.; Boulouard, M.; Dauphin, F.; Klomp, A.; Lucassen, P.J.; Homberg, J.R.; Dijkhuizen, R.M.; et al. Effects of long-term methylphenidate treatment in adolescent and adult rats on hippocampal shape, functional connectivity and adult neurogenesis. Neuroscience 2015, 309, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Ponchio, R.A.; Teodorov, E.; Kirsten, T.B.; Coelho, C.P.; Oshiro, A.; Florio, J.C.; Bernardi, M.M. Repeated methylphenidate administration during lactation reduces maternal behavior, induces maternal tolerance, and increases anxiety-like behavior in pups in adulthood. Neurotoxicology Teratol. 2015, 50, 64–72. [Google Scholar] [CrossRef]
- Huang, Z.; Hoffman, C.A.; Chelette, B.M.; Thiebaud, N.; Fadool, D.A. Elevated Anxiety and Impaired Attention in Super-Smeller, Kv1.3 Knockout Mice. Front. Behav. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Kuczenski, R.; Segal, D.S. Exposure of Adolescent Rats to Oral Methylphenidate: Preferential Effects on Extracellular Norepinephrine and Absence of Sensitization and Cross-Sensitization to Methamphetamine. J. Neurosci. 2002, 22, 7264. [Google Scholar] [CrossRef] [Green Version]
- Eisch, A.J.; Harburg, G.C. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus 2006, 16, 271–286. [Google Scholar] [CrossRef]
- Carias, E.; Fricke, D.; Vijayashanthar, A.; Smith, L.; Somanesan, R.; Martin, C.; Kalinowski, L.; Popoola, D.; Hadjiargyrou, M.; Komatsu, D.E.; et al. Weekday-only chronic oral methylphenidate self-administration in male rats: Reversibility of the behavioral and physiological effects. Behav. Brain Res. 2019, 356, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.; Gupta, S.; Guinta, D.; Flynn, D.; Agler, D.; Lerner, M.; Williams, L.; Shoulson, I.; Wigal, S. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin. Pharmacol. Ther. 1999, 66, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, L.; Connor, C.; Somanesan, R.; Carias, E.; Richer, K.; Smith, L.; Martin, C.; Mackintosh, M.; Popoola, D.; Hadjiargyrou, M.; et al. Brief and extended abstinence from chronic oral methylphenidate treatment produces reversible behavioral and physiological effects. Dev. Psychobiol. 2020, 62, 170–180. [Google Scholar] [CrossRef]
- Dafny, N.; Yang, P. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: A review of its locomotor effects. Brain Res. Bull. 2006, 68, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Fricke, D.; Vijayashanthar, A.; Lowinger, C.; Koutsomitis, D.; Popoola, D.; Hadjiargyrou, M.; Komatsu, D.E.; Thanos, P.K. Recovery from behavior and developmental effects of chronic oral methylphenidate following an abstinence period. Pharmacol. Biochem. Behav. 2018, 172, 22–32. [Google Scholar] [CrossRef]
- Robison, L.S.; Ananth, M.; Hadjiargyrou, M.; Komatsu, D.E.; Thanos, P.K. Chronic oral methylphenidate treatment reversibly increases striatal dopamine transporter and dopamine type 1 receptor binding in rats. J. Neural Transm. 2017, 124, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Mines, M.A.; Beurel, E.; Jope, R.S. Examination of methylphenidate-mediated behavior regulation by glycogen synthase kinase-3 in mice. Eur. J. Pharmacol. 2013, 698, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Uddin SM, Z.; Robison, L.S.; Fricke, D.; Chernoff, E.; Hadjiargyrou, M.; Thanos, P.K.; Komatsu, D.E. Methylphenidate regulation of osteoclasts in a dose- and sex-dependent manner adversely affects skeletal mechanical integrity. Sci. Rep. 2018, 8, 1515. [Google Scholar] [CrossRef] [Green Version]
- Warren, B.L.; Iñiguez, S.D.; Alcantara, L.F.; Wright, K.N.; Parise, E.M.; Weakley, S.K.; Bolaños-Guzmán, C.A. Juvenile Administration of Concomitant Methylphenidate and Fluoxetine Alters Behavioral Reactivity to Reward- and Mood-Related Stimuli and Disrupts Ventral Tegmental Area Gene Expression in Adulthood. J. Neurosci. 2011, 31, 10347. [Google Scholar] [CrossRef] [Green Version]
- Thanos, P.K.; McCarthy, M.; Senior, D.; Watts, S.; Connor, C.; Hammond, N.; Blum, K.; Hadjiargyrou, M.; Komatsu, D.; Steiner, H. Combined chronic oral methylphenidate and fluoxetine treatment during adolescence: Effects on behavior. Curr. Pharm. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Komatsu, D.E.; Thanos, P.K.; Mary, M.N.; Janda, H.A.; John, C.M.; Robison, L.; Ananth, M.; Swanson, J.M.; Volkow, N.D.; Hadjiargyrou, M. Chronic exposure to methylphenidate impairs appendicular bone quality in young rats. Bone 2012, 50, 1214–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.D.; Punsoni, M.; Tabori, N.E.; Melton, J.T.; Fanslow, V.; Ward, M.J.; Zupan, B.; Menzer, D.; Rice, J.; Drake, C.T.; et al. Methylphenidate Administration to Juvenile Rats Alters Brain Areas Involved in Cognition, Motivated Behaviors, Appetite, and Stress. J. Neurosci. 2007, 27, 7196–7207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Teylan, M.A.; Baron, M.; Sands, A.; Nairn, A.C.; Greengard, P. Methylphenidate-induced dendritic spine formation and ΔFosB expression in nucleus accumbens. Proc. Natl. Acad. Sci. USA 2009, 106, 2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schermann, H.; Gurel, R.; Ankory, R.; Kadar, A.; Yoffe, V.; Snir, N.; Sternheim, A.; Karakis, I. Lower risk of fractures under methylphenidate treatment for ADHD: A dose-response effect. J. Orthop. Res. 2018, 36, 3328–3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delis, F.; Weber, A.; Thanos, P. Chronic oral methylphenidate intake affects white matter morphology and NDMA recepor density in normal rats. In Proceedings of the 27th Meeting of the Hellenic Society for Neuroscience, Athens, Greece, 8–10 December 2017. [Google Scholar]
- Rapport, M.D.; Moffitt, C. Attention deficit/hyperactivity disorder and methylphenidate. A review of height/weight, cardiovascular, and somatic complaint side effects. Clin. Psychol. Rev. 2002, 22, 1107–1131. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.; Greenhill, L.; Wigal, T.; Kollins, S.; Stehli, A.; Davies, M.; Chuang, S.; Vitiello, B.; Skrobala, A.; Posner, K.; et al. Stimulant-related reductions of growth rates in the PATS. J. Am. Acad. Child. Adolesc. Psychiatry 2006, 45, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Chirokikh, A.A.; Uddin, S.M.Z.; Areikat, N.; Jones, R.; Duque, E.; Connor, C.; Hadjiargyrou, M.; Thanos, P.K.; Komatsu, D.E. Combined methylphenidate and fluoxetine treatment in adolescent rats significantly impairs weight gain with minimal effects on skeletal development. Bone 2023, 167, 116637. [Google Scholar] [CrossRef]
- Wilens, T.E. Effects of Methylphenidate on the Catecholaminergic System in Attention-Deficit/Hyperactivity Disorder. J. Clin. Psychopharmacol. 2008, 28, S46–S53. [Google Scholar] [CrossRef]
- Martinez, E.; Pasquereau, B.; Drui, G.; Saga, Y.; Météreau, É.; Tremblay, L. Ventral striatum supports Methylphenidate therapeutic effects on impulsive choices expressed in temporal discounting task. Sci. Rep. 2020, 10, 716. [Google Scholar] [CrossRef] [Green Version]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialog. Clin. Neurosci. 2000, 2, 219–232. [Google Scholar] [CrossRef]
- Jalloh, K.; Roeder, N.; Hamilton, J.; Delis, F.; Hadjiargyrou, M.; Komatsu, D.; Thanos, P.K. Chronic oral methylphenidate treatment in adolescent rats promotes dose-dependent effects on NMDA receptor binding. Life Sci. 2021, 264, 118708. [Google Scholar] [CrossRef] [PubMed]
- Connor, C.; Hamilton, J.; Robison, L.; Hadjiargyrou, M.; Komatsu, D.; Thanos, P. Abstinence from Chronic Methylphenidate Exposure Modifies Cannabinoid Receptor 1 Levels in the Brain in a Dose-dependent Manner. Curr. Pharm. Des. 2022, 28, 331–338. [Google Scholar] [CrossRef]
- Richer, K.; Hamilton, J.; Delis, F.; Martin, C.; Fricke, D.; Yao, R.; Sajjad, M.; Blum, K.; Hadjiargyrou, M.; Komatsu, D.; et al. Chronic treatment and abstinence from methylphenidate exposure dose-dependently changes glucose metabolism in the rat brain. Brain Res. 2022, 1780, 147799. [Google Scholar] [CrossRef] [PubMed]
- Arnavut, E.; Hamilton, J.; Yao, R.; Sajjad, M.; Hadjiargyrou, M.; Komatsu, D.; Thanos, P.K. Abstinence following intermittent methylphenidate exposure dose-dependently modifies brain glucose metabolism in the rat brain. Synapse 2022, 76, 17–30. [Google Scholar] [CrossRef] [PubMed]
| Developmental Effects | Model Used/References | Behavioral Effects | Model Used/References |
|---|---|---|---|
| Food intake: Rats exposed to MP were prone to increase in food intake when compared to the control group. | Sprague-Dawley rats [85] | Open field locomotor activity: Rats exposed to MP have increased locomotor activity. Rats injected with MP effected faster than by MP Oral voluntary. | Sprague-Dawley rats [14] Sprague-Dawley rats [51] |
| Body Weight: Rats exposed to MP tend to have decreased body weight when compared to the control group. Body weights tend to decrease over time, with more significant decreases in the later weeks of treatment, espescially in females. | Sprague-Dawley rats [85] Sprague-Dawley rats [14] | Sleep and Circadian: Both male and female experienced more activity than water rats during the dark cycle. Females experienced more activity than male. | Sprague-Dawley rats [86] |
| Skeletal Effects: Rats exposed to MP demonstrated an increase in osteoclast formation on the surface of the cortical bone. Shown to experience a decrease in skeletal growth and mineralization. Decrease in stress fractures Some studies indicate growth suppression. | Sprague-Dawley rats [88] Sprague-Dawley rats [91] Human children [94] | Anxiety: Rats exposed to MP are shown to have decreased levels of anxiety. Male experience less anxiety than female rats due to biological differences. | Neonatal 6-hydroxydopamine mice [81] Sprague-Dawley rats [86] |
| Depression: MP rats displayed a >latency to immobility compared to water-treated rats. Rats showed increased signs of depressive symptoms. | Sprague-Dawley rats [81] | ||
| Memory: Some studies showed an increase in cognitive malfunction in rats exposed to MP. Other studies showed distruption in novel object exploration as a result in altered memory in rats exposed to MP. | Neonatal 6-hydroxydopamine mice [52] Sprague-Dawley rats [86] | ||
| Cocaine-Conditioned Place Preference: A higher dose of cocaine demonstrates greater drug-seeking behavior. There is no cohesive trend of MP exposure promoting Cocaine CPP. | Sprague-Dawley rats [63] |
| Arnavut et al., 2022 [105] | Richer et al., 2022 [104] | |
|---|---|---|
| Treatment phase: LD MP > Control | None | Hippocampus and subiculum and simple lobule |
| Treatment phase: HD MP > Control | None | Sensorimotor cortex, primary auditory cortex, ectorhinal cortex, inferior colliculus, and temporal association cortex |
| Treatment phase: HD MP > LD MP | Primary and secondary visual cortex Ectorhinal cortex | Cingulate cortex and striatum |
| Abstinence phase: 1 week LD MP > Control | None | Medial orbital cortex, lateral hypothalamus, hippocampus, and subiculum |
| Abstinence phase: 1 week HD MP > Control | Superior cerebellar peduncle, tectospinal tract, sagulum nucleus and lateral lemniscus Inferior colliculus, caudomedial entorhinal field, pedunculopontine tegmental nucleus, 5th and 6c cerebellar lobule, cerebellar nucleus and cerebellar white matter | None |
| Abstinence phase: 1 week HD MP > LD MP | Sagulum nucleus, lateral lemniscus, external cortex of the inferior colliculus, caudomedial entorhinal field, spinal trigeminal nucleus, dorsomedial spinal trigeminal nucleus, parvicellular reticular nucleus | Medial orbital cortex, insular cortex, basal/lateral amygdaloid nucleus, dorsal endopiriform nucleus, lateral amygdaloid nucleus and pontine reticular nucleus |
| Abstinence phase: 4 weeks LD MP > Control | None | None |
| Abstinence phase: 4 weeks HD MP > Control | Mesencephalic reticular formation, lateral and medial lemniscus, microcellular tegmental nucleus, inferior colliculus Inferior olive, trigeminal nucleus and nerve trapezoid body, motor trigeminal nucleus and nerve, cerebellar white matter, crus 2 of the ansiform lobule, 8th cerebellar lobule, reticular nucleus, spinal trigeminal, and pyramidal tract | Hippocampus, retrosplenial cortex |
| Abstinence phase: 4 weeks HD MP > LD MP | Preoptic area, hypothalamic area, parasubiculum, inferior colliculus, retrosplenial dysgranular cortex, trigeminal nucleus, medioventral periolivary nucleus, trapezoid body, pyramidal tract, and gigantocellular reticular nucleus | Cuneiform nucleus |
| Abstinent phase 1 week LD MP > Treatment phase LD MP | None | Nucleus accumbens, striatum, lateral hypothalamus, hippocampus, solitary nucleus |
| Treatment phase HD MP < Abstinent phase 1 week HD MP | None | Ventral and medial orbital cortex |
| Treatment phase LD MP > Abstinent phase 4 weeks LD MP | None | Striatum, insular cortex |
| Treatment phase HD MP > Abstinent phase 4 weeks HD MP | None | Ventral and lateral orbital cortex & anterior olfactory nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senior, D.; Ahmed, R.; Arnavut, E.; Carvalho, A.; Lee, W.X.; Blum, K.; Komatsu, D.E.; Hadjiargyrou, M.; Badgaiyan, R.D.; Thanos, P.K. Behavioral, Neurochemical and Developmental Effects of Chronic Oral Methylphenidate: A Review. J. Pers. Med. 2023, 13, 574. https://doi.org/10.3390/jpm13040574
Senior D, Ahmed R, Arnavut E, Carvalho A, Lee WX, Blum K, Komatsu DE, Hadjiargyrou M, Badgaiyan RD, Thanos PK. Behavioral, Neurochemical and Developmental Effects of Chronic Oral Methylphenidate: A Review. Journal of Personalized Medicine. 2023; 13(4):574. https://doi.org/10.3390/jpm13040574
Chicago/Turabian StyleSenior, Daniela, Rania Ahmed, Eliz Arnavut, Alexandra Carvalho, Wen Xuan Lee, Kenneth Blum, David E. Komatsu, Michael Hadjiargyrou, Rajendra D. Badgaiyan, and Panayotis K. Thanos. 2023. "Behavioral, Neurochemical and Developmental Effects of Chronic Oral Methylphenidate: A Review" Journal of Personalized Medicine 13, no. 4: 574. https://doi.org/10.3390/jpm13040574