Tubular Scaffold with Shape Recovery Effect for Cell Guide Applications
Abstract
:1. Introduction
2. Results
2.1. Morphological Properties



| Sample | Porosity (Area %) |
|---|---|
| PLA-PVAc | 17.1 |
| PLA-15% β-TCP | 25.0 |
| PLA-30% β-TCP | 25.3 |
2.2. Mechanical Properties

2.3. Swelling Properties


2.4. Shape Recovery Properties

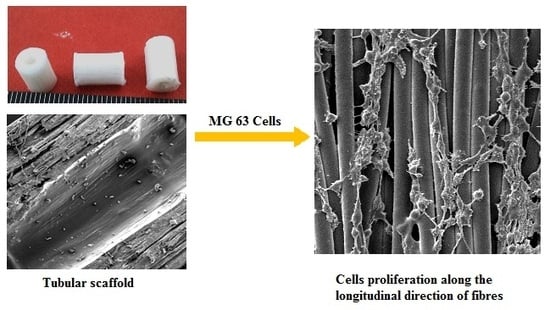
2.5. Cell Study

3. Discussion

4. Experimental Section
4.1. PLA Fibre Drawing and Production of Fibre Mat
4.2. Preparation of Coating Materials
| Sample Codes Used in This Study | Composition of Coating Blends | Overall Coating Materials Deposited within the Tubular Scaffolds (Obtained after Drying the Tubes at 37 °C for 48 h) (wt %) | |||
|---|---|---|---|---|---|
| PVAc (g) | β-TCP (g) | Deionised Water (mL) | Concentration of β-TCP in the Coating Suspension (wt %) | ||
| PLA PVAc | 1 | – | 100 | 0 | 28 ± 2 |
| PLA-15% β-TCP | 0.85 | 0.15 | 100 | 15 | 27 ± 1 |
| PLA-30% β-TCP | 0.70 | 0.30 | 100 | 30 | 29 ± 2 |
4.3. Manufacture of PLA Fibre Mats and Tubular Scaffolds

4.4. Characterisation
4.4.1. Scanning Electron Microscopic (SEM) Analysis
4.4.2. Microcomputed Tomography (μCT)
4.4.3. Compression Properties
4.4.4. Swelling Properties in Phosphate Buffer Saline (PBS) Media
4.4.5. Cell attachment and Morphology Assessment
4.4.6. Statistical Analysis
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, R.L.; Neves, N.M.; Mano, J.F.; Gomes, M.E.; Marques, A.P.; Azevedo, H.S. Natural-Based Polymers for Biomedical Applications; CRC Press LLC: New York, NY, USA, 2008. [Google Scholar]
- Narayan, R. Biomedical Materials; Springer: New York, NY, USA, 2009. [Google Scholar]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef]
- Jones, J.R.; Gentleman, E.; Polak, J. Bioactive glass scaffolds for bone regeneration. Elements 2007, 3, 393–399. [Google Scholar] [CrossRef]
- Liu, X.; Rahaman, M.N.; Hilmas, G.E.; Bal, B.S. Mechanical properties of bioactive glass (13–93) scaffolds fabricated by robotic deposition for structural bone repair. Acta Biomater. 2013, 9, 7025–7034. [Google Scholar] [CrossRef] [PubMed]
- Nachtrab, S.; Kapfer, S.C.; Rietzel, D.; Drummer, D.; Madadi, M.; Arns, C.H.; Kraynik, A.M.; Schröder-Turk, G.E.; Mecke, K. Tuning elasticity of open-cell solid foams and bone scaffolds via randomized vertex connectivity. Adv. Eng. Mater. 2012, 14, 120–124. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, L.; Li, W.; Yao, D. Fabrication of tissue engineering scaffolds through solid-state foaming of immiscible polymer blends. Biofabrication 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Lin, S.; Yue, S.; Lee, P.D.; Hanna, J.V.; Smith, M.E.; Newport, R.J. Bioactive glass scaffolds for bone regeneration and their hierarchical characterisation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1373–1387. [Google Scholar] [CrossRef]
- Day, R.; Boccaccini, A.; Maquet, V.; Shurey, S.; Forbes, A.; Gabe, S.; Jérôme, R. In vivo characterisation of a novel bioresorbable poly(lactide-co-glycolide) tubular foam scaffold for tissue engineering applications. J. Mater. Sci. Mater. Med. 2004, 15, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Slotosch, A.; Thibaudeau, L.; Taubenberger, A.; Loessner, D.; Vaquette, C.; Dalton, P.; Hutmacher, D. Design and fabrication of tubular scaffolds via direct writing in a melt electrospinning mode. Biointerphases 2012, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Heo, D.N.; Park, J.S.; Kwon, S.K.; Lee, J.H.; Lee, J.H.; Kim, W.D.; Il Keun, K.; Park, S.A. Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular by combining electrospinning and 3D printing system. Phys. Chem. Chem. Phys. 2015, 17, 2996–2999. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-Y.; Paik, D.-H.; Choi, S.-W. Fabrication of tubular scaffolds with controllable fiber orientations using electrospinning for tissue engineering. Macromol. Mater. Eng. 2014, 299, 1425–1429. [Google Scholar] [CrossRef]
- Bashur, C.A.; Ramamurthi, A. Aligned electrospun scaffolds and elastogenic factors for vascular cell-mediated elastic matrix assembly. J. Tissue Eng. Regen. Med. 2012, 6, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M.; Miot, S.; Gorecka, A.; Singha, K.; Loparic, M.; Dickinson, S.; Das, A.; Bhavesh, N.S.; Ray, A.R.; Martin, I.; et al. Oriented lamellar silk fibrous scaffolds to drive cartilage matrix orientation: Towards annulus fibrosus tissue engineering. Acta Biomater. 2012, 8, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Huang, X.; Li, Z.; Song, L.; Wang, H.; Xu, Y.; Shao, H.; Zhang, Y. Silk fibroin tissue engineering scaffolds with aligned electrospun fibers in multiple layers. RSC Adv. 2014, 4, 47570–47575. [Google Scholar] [CrossRef]
- Gérard, C.; Doillon, C.J. Facilitating tissue infiltration and angiogenesis in a tubular collagen scaffold. J. Biomed. Mater. Res. Part A 2010, 93A, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Hoogenkamp, H.R.; Koens, M.J.; Geutjes, P.J.; Ainoedhofer, H.; Wanten, G.; Tiemessen, D.M.; Hilborn, J.; Gupta, B.; Feitz, W.F.; Daamen, W.F.; et al. Seamless vascularized large-diameter tubular collagen scaffolds reinforced with polymer knittings for esophageal regenerative medicine. Tissue Eng. Part C Methods 2014, 20, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Aframian, D.J.; Redman, R.S.; Yamano, S.; Nikolovski, J.; Cukierman, E.; Yamada, K.M.; Kriete, M.F.; Swaim, W.D.; Mooney, D.J.; Baum, B.J. Tissue compatibility of two biodegradable tubular scaffolds implanted adjacent to skin or buccal mucosa in mice. Tissue Eng. 2002, 8, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, G.; Nitta, N.; Matsuda, S.; Sakamoto, Y.; Isayama, N.; Yamazaki, K.; Ikada, Y. Long-term results of cell-free biodegradable scaffolds for in situ tissue-engineering vasculature: In a canine inferior vena cava model. PLoS ONE 2012, 7, e35760. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.; Oka, M. Collagen filaments as a scaffold for nerve regeneration. J. Biomed. Mater. Res. 2001, 56, 400–405. [Google Scholar] [CrossRef]
- Biazar, E.; Khorasani, M.T.; Montazeri, N.; Pourshamsian, K.; Daliri, M.; Rezaei, M.; Jabarvand, M.; Khoshzaban, A.; Heidari, S.; Jafarpour, M.; et al. Types of neural guides and using nanotechnology for peripheral nerve reconstruction. Int. J. Nanomed. 2010, 5, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Cunha, C.; Lowery, J.; Del Carro, U.; Taraballi, F.; Amadio, S.; Vescovi, A.; Gelain, F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.A.; Hastings, A.Z.; Yannas, I.V.; Sannino, A. Fabricating tubular scaffolds with a radial pore size gradient by a spinning technique. Biomaterials 2006, 27, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Balian, G.; Chhabra, A.B. Tendon tissue engineering and gene transfer: The future of surgical treatment. J. Hand Surg. 2006, 31, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, N.; Fujioka, H.; Kokubu, T.; Nagura, I.; Inui, A.; Sakata, R.; Satake, M.; Kaneko, H.; Kurosaka, M. Electrospun synthetic polymer scaffold for cartilage repair without cultured cells in an animal model. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsson, A.; Stenhamre, H.; Gatenholm, P.; Walkenström, P. Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules 2008, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Ekaputra, A.K.; Zhou, Y.; Cool, S.M.; Hutmacher, D.W. Composite electrospun scaffolds for engineering tubular bone grafts. Tissue Eng. Part A 2009, 15, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Causa, F.; Taddei, P.; di Foggia, M.; Ciapetti, G.; Martini, D.; Fagnano, C.; Baldini, N.; Ambrosio, L. Polylactic acid fibre-reinforced polycaprolactone scaffolds for bone tissue engineering. Biomaterials 2008, 29, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Soffer, L.; Wang, X.; Zhang, X.; Kluge, J.; Dorfmann, L.; Kaplan, D.L.; Leisk, G. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 2008, 19, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.M.; van Tuijl, S.; Bouten, C.V.C.; Baaijens, F.P.T. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005, 1, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, J.; Chu, C.-C.; Wu, J. Electrospinning of small diameter 3-D nanofibrous tubular scaffolds with controllable nanofiber orientations for vascular grafts. J. Mater. Sci. Mater. Med. 2010, 21, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Den Dunnen, W.F.A.; van der Lei, B.; Schakenraad, J.M.; Stokroos, I.; Blaauw, E.; Bartels, H.; Pennings, A.J.; Robinson, P.H. Poly(DL-lactide-epsilon-caprolactone) nerve guides perform better than autologous nerve grafts. Microsurgery 1996, 17, 348–357. [Google Scholar] [CrossRef]
- Ngo, T.-T.B.; Waggoner, P.J.; Romero, A.A.; Nelson, K.D.; Eberhart, R.C.; Smith, G.M. Poly(l-lactide) microfilaments enhance peripheral nerve regeneration across extended nerve lesions. J. Neurosci. Res. 2003, 72, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Rangappa, N.; Romero, A.; Nelson, K.D.; Eberhart, R.C.; Smith, G.M. Laminin-coated poly(l-lactide) filaments induce robust neurite growth while providing directional orientation. J. Biomed. Mater. Res. 2000, 51, 625–634. [Google Scholar] [CrossRef]
- Widmer, M.S.; Gupta, P.K.; Lu, L.; Meszlenyi, R.K.; Evans, G.R.D.; Brandt, K.; Savel, T.; Gurlek, A.; Patrick, C.W., Jr.; Mikos, A.G. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials 1998, 19, 1945–1955. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Parsons, A.J.; Rudd, C.D.; Ahmed, I.; Thielemans, W. Mechanical, crystallisation and moisture absorption properties of melt drawn polylactic acid fibres. Eur. Polym. J. 2014, 53, 270–281. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Hasan, M.S.; Boyd, D.; Rudd, C.D.; Ahmed, I.; Thielemans, W. Effect of cellulose nanowhiskers on surface morphology, mechanical properties, and cell adhesion of melt-drawn polylactic acid fibers. Biomacromolecules 2014, 15, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.Z.; Felfel, R.M.; Rudd, C.D.; Thielemans, W.; Ahmed, I. The effect of cellulose nanowhiskers on the flexural properties of self-reinforced polylactic acid composites. React. Funct. Polym. 2014, 85, 193–200. [Google Scholar] [CrossRef]
- Shimko, D.A.; Shimko, V.F.; Sander, E.A.; Dickson, K.F.; Nauman, E.A. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Jones, F.R. A review of particulate reinforcement theories for polymer composites. J. Mater. Sci. 1990, 25, 4933–4942. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-l-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.E.; Capadona, J.R.; Shanmuganathan, K.; Hsu, L.; Rowan, S.J.; Weder, C.; Tyler, D.J.; Zorman, C.A. Development of a stimuli-responsive polymer nanocomposite toward biologically optimized, mems-based neural probes. J. Micromech. Microeng. 2011, 21. [Google Scholar] [CrossRef]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yi, S.; Li, H.; Huang, C. Preparation of poly(vinyl acetate) modified by triethoxyvinylsilane and properties of copolymeric lattices. Iran. Polym. J. 2007, 16, 207–213. [Google Scholar]
- Kolter, K.; Dashevsky, A.; Irfan, M.; Bodmeier, R. Polyvinyl acetate-based film coatings. Int. J. Pharm. 2013, 457, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Shen, C.-C.; Cheng, H.-C.; Liu, B.-S. Sciatic nerve repair by reinforced nerve conduits made of gelatin-tricalcium phosphate composites. J. Biomed. Mater. Res. Part A 2011, 96, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Shen, C.-C.; Huang, T.-B.; Chang, S.-H.; Cheng, H.-C.; Liu, B.-S. Characteristics and biocompatibility of a biodegradable genipin-cross-linked gelatin/β-tricalcium phosphate reinforced nerve guide conduit. J. Biomed. Mater. Res. Part B Appl. Biomateri. 2010, 95, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-C.; Yang, Y.-C.; Liu, B.-S. Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury 2011, 42, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.Z.; Jasmani, L.; Ahmed, I.; Parsons, A.J.; Scotchford, C.A.; Thielemans, W.; Rudd, C.D. High cellulose nanowhisker content composites through cellosize bonding. Soft Matter 2012, 8, 12099–12110. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, K.M.Z.; Zhu, C.; Felfel, R.M.; Sharmin, N.; Ahmed, I. Tubular Scaffold with Shape Recovery Effect for Cell Guide Applications. J. Funct. Biomater. 2015, 6, 564-584. https://doi.org/10.3390/jfb6030564
Hossain KMZ, Zhu C, Felfel RM, Sharmin N, Ahmed I. Tubular Scaffold with Shape Recovery Effect for Cell Guide Applications. Journal of Functional Biomaterials. 2015; 6(3):564-584. https://doi.org/10.3390/jfb6030564
Chicago/Turabian StyleHossain, Kazi M. Zakir, Chenkai Zhu, Reda M. Felfel, Nusrat Sharmin, and Ifty Ahmed. 2015. "Tubular Scaffold with Shape Recovery Effect for Cell Guide Applications" Journal of Functional Biomaterials 6, no. 3: 564-584. https://doi.org/10.3390/jfb6030564