Pullulan for Advanced Sustainable Body- and Skin-Contact Applications
Abstract
:1. Introduction
2. Polysaccharides and Waste
3. Exopolysaccharide Synthesis and Applications
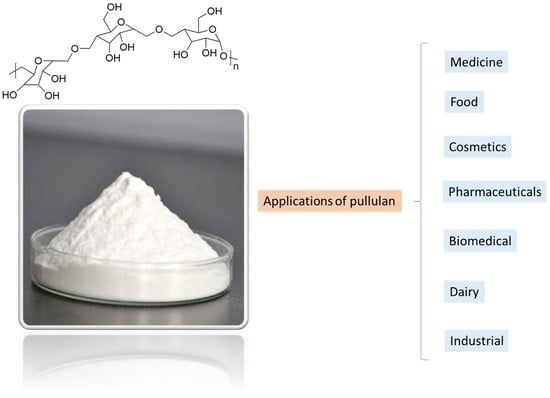
4. Pullulan Characteristics, Applications, and Market
5. Production, Biodegradation, and Biomedical Applications of Pullulan
6. Pullulan and Nanotechnology
7. Pullulan and Extra Cellular Matrix
8. Skin Structure and Skin Contact Applications
9. Pullulan and Personal Care/Cosmetic Market
10. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singhal, R.S.; Kulkarni, P.R. Production of food Additives by fermentation. In Biotechnology Food Fermentation; Joshi, V.K., Pandey, A., Eds.; Asiatech Publishers Inc.: New Delhi, India, 1999; pp. 144–200. [Google Scholar]
- Youssef, F.; Roukas, T.; Biliaderis, G.G. Pullulan production by a non-pigmented strain of Aureobasidium pullulans using batch and fedbatch culture. Process. Biochem. 1999, 34, 355–366. [Google Scholar] [CrossRef]
- Shukla, A.; Mehta, K.; Parmar, J.; Pandya, J.; Saraf, M. Depicting the Exemplary Knowledge of Microbial Exopolysaccharides in a Nutshell. Eur. Polym. J. 2019, 119, 298–310. [Google Scholar] [CrossRef]
- Kumar, D.; Saini, N.; Pandit, V.; Ali, S. An Insight to Pullulan: A Biopolymer in Pharmaceutical Approaches. Int. J. Basic Appl. Sci. 2012, 1, 202–219. [Google Scholar] [CrossRef]
- Haniffa, M.; Cader, M.A.; Ching, Y.C.; Abdullah, L.C.; Poh, S.C.; Chuah, C.H. Review of Bionanocomposite Coating Films and Their Applications. Polymers 2016, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, L.; Tomasula, P.; Moreira de Sousa, A.M.; Kevin, L. Electrospinning Pullulan Fibers from Solutions. Polymers 2017, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef] [PubMed]
- FDA: US Food and Drug Administration, Center for Food and Applied Nutrition, Office of Food Safety, Agency Response Letter, GRAS Notice No. GRN 000099. Available online: https://www.ams.usda.gov/sites/default/files/media/PullulanPetition18131.pdf (accessed on 13 March 2020).
- Alhaique, F.; Matricardi, P.; Di Meo, C.; Coviello, T.; Montanari, E. Polysaccharide-based self-assembling nanohydrogels: An overview on 25-years research on pullulan. J. Drug Deliv. Sci. Technol. Part B 2015, 30, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Tabasum, S.; Noreen, A.; Farzam Maqsood, M.; Umar, H.; Akram, N.; Nazli, Z.; Chatha, S.A.S.; Zia, K.M. A review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. Int. J. Biol. Macromol. Part A 2018, 120, 603–632. [Google Scholar] [CrossRef]
- Badwhar, P.; Dubey, K.K. Insights of Microbial Pullulan Production: A Bioprocess Engineer Assessment. Curr. Biotechnol. 2018, 7, 262–272. [Google Scholar] [CrossRef]
- Grigoras, A.G. Drug delivery systems using pullulan, a biocompatible polysaccharide produced by fungal fermentation of starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, R.; Yang, S.; Guan, J.; Zhang, D.; Ma, Y.; Liu, H. Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 2018, 25, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Sharma, P.; Katti, D.S. Pullulan-based composite scaffolds for bone tissue engineering: Improved osteoconductivity by pore wall mineralization. Carbohydr. Polym. 2015, 123, 180–189. [Google Scholar]
- San Juan, A.; Hlawaty, H.; Chaubet, F.; Letourneur, D. Cationized pullulan 3D as new materials for gene transfer. J. Biomed. Mat. Res. Part A 2007, 82, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue enginnering applications. Adv. Drug Deliv. 2007, 59, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Hanson, S.; Hematti, P. Clinical Applications of Mesenchymal Stem Cell-Bionanomaterial Constructs for Tissue Reconstruction. In Biomaterials in Regenerative Medicine; Ramalingam, M., Ramakrishna, S., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 479–491. [Google Scholar]
- Nair, L.S.; Laurencin, C. Biodegradable polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Seidi, A.; Sampathkumar, K.; Srivastava, A.; Ramakrishna, S.; Ramalingam, M. Gradient Nanofiber Scaffolds for Tussue Engineering. J. Nanosci. Nanotechnol. 2013, 13, 4647–4655. [Google Scholar] [CrossRef]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef]
- Farris, S.; Unalan, I.U.; Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A. Pullulan-Based Films and Castings for Food Packagings: Present Applications, Emergjng Opportunities and Future Challenges. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Coltelli, M.B. A new Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [Green Version]
- Padmaja, G.; Jyoth, A.N. Roots and Tubers. In Valorization of Food Processing by-Products; Chandrasekaran, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 377–414. [Google Scholar]
- Heinze, T. (Ed.) Polysaccharydes: Structure, Characterization and Use; Springer: New York, NY, USA, 2005. [Google Scholar]
- Hou, Y.; Ding, X.; Hou, W. Composition and antioxidant activity of water-soluble oligosaccharides from Hericium erinaceus. Mol. Med. Rep. 2015, 11, 3794–3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desnpande, M.S.; Rale, V.B.; Lynch, J. Aureobasidium pullulans in applied microbiology: A status report. Enzym. Microbial Technol. 1992, 14, 514–527. [Google Scholar] [CrossRef]
- Morganti, P. Use of Chitin Nanofibrils from Biomass for an Innovative Bioeconomy. In Nanofabrication Using Nanomaterials; Ebothe, J., Ahmed, W., Eds.; One Central Press: Manchester, UK, 2016; pp. 1–22. [Google Scholar]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rather, I.A.; Majumder, R.; Shunkla, S.; Aeron, A.; Kim, K.; Kang, S.C.; Dubey, R.C.; Maheshwari, D.K.; Lim, J.; et al. Exopolisaccharyde and lactic acid bacteria: Perception, functionality and prospects. Bangladesh J. Pharmacol. 2020, 1, 1–23. [Google Scholar] [CrossRef]
- Rabha, B.; Nadra, R.S.; Amhed, B. Effect of some fermentation substrates and growth temperature on Exopolysaccharide production by Streptococcus thermophilus BN1. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 44. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides-a perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial sources, production and Applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef]
- Oguzhan, P.; Yangilar, F. Pullulan: Production and usage in food industry. Afr. J. Food Sci. Technol. 2013, 4, 57–63. [Google Scholar]
- Rhim, J.W. Characteristics of Pullulan based Edible films. Food Sci. Bitechnol. 2003, 12, 161–165. [Google Scholar]
- Sugumaran, K.R.; Ponnusami, V. Review on production, downstream processing and characterization of microbial Pullulan. Carbohydr. Polym. 2017, 173, 573–591. [Google Scholar]
- Pullulan Market 2019 Industry Research, Share, Trend, Global Industry Side, Price, Future Analysis, Regional Outlook to 2024. Available online: https://www.marketwatch.com/press-release/pullulan-market-2019-industry-size-and-share-evolution-to-2024-by-growth-insight-key-development-trends-and-forecast-by-market-reports-world-2019-07-24 (accessed on 11 March 2020).
- Morganti, P.; Coltelli, M.B.; Danti, S. Biobased Tissues for Innovative Cosmetic Products: Polybioskin as an EU Reesrch Project. Glob. J. Nanomed. 2018, 3, 555620. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Coltelli, M.B. Chitin Nanomaterials and Nanocompositrs for Tissue Repair. In Marine-Derived Biomateriaks for Tissue Engineeing Applications; Choi, A.H., Ben-Nissan, B., Eds.; Springer: Singapore, 2019; pp. 523–544. [Google Scholar]
- Morganti, P.; Danti, S.; Coltelli, M.B. Chitin and lignin to produce biocompatible tissues. Res. Clin. Dermatol. 2018, 1, 5–11. [Google Scholar] [CrossRef]
- Schlaubitz, S.; Derkaoui, S.M.; Marosa, L.; Miraux, S.; Renard, M.; Catros, S.; Le Visage, C.; Letourneur, D.; Amédée, J.; Fricain, J.C. Pullulan/dextran/nHA Macroporous Composite Beads for Bone Repair in a Femoral Condyle Defect in Rats. PLoS ONE 2014, 9, e110251. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; Schlaubitz, S.; Le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A nano-hydroxyapatite—Pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef] [PubMed]
- Takahata, T.; Okihara, T.; Yoshida, Y.; Yoshihara, K.; Shiozaki, Y.; Yoshida, A.; Yamane, K.; Watanabe, N.; Yoshimura, M.; Nakamura, M.; et al. Bone engineering by phosphorylated-pullulan and b-TCP composite. Biomed. Mater. 2015, 10, 65009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydogdu, H.; Keskin, D.; Baran, E.T.; Tezcaner, A. Pullulan microcarriers for bone tissue regeneration. Mater. Sci. Eng. C 2016, 63, 439–449. [Google Scholar] [CrossRef]
- Donota, A.; Fontana, J.C.; Schorr-Galindo, B.S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M.; Pandey, A. Biodegradation of Biopolymers. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 739–755. [Google Scholar]
- Morganti, P.; Morganti, G.; Colao, C. Biofunctioal Textiles for Aged Skin. Biomedicines 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Bruneel, D.; Schacht, E. Enzymatic Degradation of Pullulan and Pullulan Derivatives. J. Bioact. Compat. Polym. 1995, 10, 299–312. [Google Scholar] [CrossRef]
- Mishra, B.; Vuppu, S.; Rath, K. The role of microbial pullulan, a biopolymer in pharmaceutical approaches: A review. J. Appl. Pharm. Sci. 2011, 1, 45–50. [Google Scholar]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Della Giustina, G.; Gandin, A.; Brigo, L.; Panciera, T.; Giulitti, S.; Sgarbossa, P.; D’Alessandro, D.; Trombi, L.; Danti, S.; Brusatin, G. Polysaccharide hydrogels for multiscale 3D printing of pullulan scaffolds. Mater. Des. 2019, 165, 107566. [Google Scholar] [CrossRef]
- Singh, R.M.; Kaur, N.; Rana, V.; Kennedy, J.K. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef]
- Alma Khan, F. (Ed.) Biotechnology Fundamentals; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Nochi, T.; Yuki, Y.; Takahashi, H. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010, 9, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Rodrigues, S. Pullulan-based nanoparticles: Future therapeutic applications in transmucosal protein delivery. Ther. Deliv. 2013, 4, 1339–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilizationof insulin. J. Control. Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Huang, L. Versatile redox-sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1005–1017. [Google Scholar] [CrossRef]
- Hong, L.; Kim, W.-S.; Lee, S.-M.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Pullulan Nanoparticles as Prebiotics Enhance the Antibacterial Properties of Lactobacillus plantarum Through the Induction of Mild Stress in Probiotics. Front. Microbiol. 2019, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Giri, A.; Goswami, N.; Sarkar, S.; Pal, S.K. Bio-Nanomaterials: Understanding Key Biophysics and their Applications. Nanotechnology 2013, 11, 41–110. [Google Scholar] [CrossRef]
- Abedini, F.; Ebrahimi, M.; Roozbehani, A.H.; Domb, A.J.; Hosseinkhani, H. Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polym. Adv. Technol. 2018, 29, 2564–2573. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Li, Y.; Ma, X.; Ju, J.; Sun, X.; Deng, N.; Li, Z.; Kang, W.; Cheng, B. Preparation and characterization of crisslimked electrospun nanofiber membrane as a potential for biomaterial. J. Text. Inst. 2018, 109, 756–759. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water: Formation and characteristic of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Khan, F.; Ahmed, S.R. Fabrication of 3D Scaffolds on Organ Printing for Tisshe Regeneration. In Biomaterials and Stem Cells in Regenerative Medicine; Ramalingam, M., Ramakrishna, S., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 101–122. [Google Scholar]
- Kong, L.; Ziegler, G.R. Rheological aspects in fabricating Pululan fibers by electro-wet-spinning. Food Hydrocoll. 2014, 38, 226–229. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Torre-Muruzabal, A.; Daelemans, L.; Van Assche, G.; De Clerck, K.; Rahier, H. Creation of a nanovascular network by electrospun sacrificial nanofibers fors elf-healing applications and its effect on the flexural properties of the bulk material. Polym. Test. 2016, 54, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Jia, D.; Kang, W.; Cheng, B.; Li, Y. Research on electrospinning process of pullulan nanofibers. Appl. Mech. Mater. 2013, 268–270, 198–201. [Google Scholar] [CrossRef]
- Karim, M.R.; Lee, H.W.; Kim, R.; Ji, B.C.; Cho, J.W.; Son, T.W.; Oh, W.; Yeum, J.H. Preparation and characterization of electrospun pullulan/montmorillonite nanofiber mats in aqueous solution. Carbohydr. Polym. 2009, 78, 336–342. [Google Scholar] [CrossRef]
- Qian, Y.; Qi, M.; Zheng, L.; King, M.W.; Lyu, L.; Ye, F. Incorporation of Rutin in Electrospun Pullulan/PVA Nanofibers for Novel UV-Resistant Properties. Materials 2016, 9, 504. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ziegler, G.R. Electrospun nanofiber mats from aqueous starch-pullulan dispersions: Optimizing dispersion properties for electrospinning. Int. J. Biol. Macromol. 2019, 133, 1168–1174. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Qian, Y.; Zhang, Z.; Lyu, L.; Wang, Y.; Ye, F. Study on the Electrospinning of Gelatin/Pullulan Composite Nanofibers. Polymers 2019, 11, 1424. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Del Ciotto, P.; Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Anti-Inflammatory, Immunomodulatory, and Tissue Repair Activity on Human Keratinocytes by Green Innovative Nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef] [Green Version]
- Priestley, G.C. (Ed.) Molecular Aspects of Dermatology; John Wiley & Sons Ltd.: Chichester, UK, 1993. [Google Scholar]
- Rawlings, A.V.; Leyden, J.J. (Eds.) Skin Moisturizing, 2nd ed.; Informa Healthcare: New York, NY, USA, 2009. [Google Scholar]
- Prausnitz, M.R.; Mitragotri, S.; Lager, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2008, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.G.; Jungvid, H.; Kumar, A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng. Part B Rev. 2008, 14, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Rustad, K.C.; Gálvez, M.G.; Neofytou, E.; Glotzbach, J.P.; Hanuszy, K.; Major, M.R. Engineered Pullulan Collagen Composite Dermatol Hydrogels Improve Early Cutaneous Wiund Healing. Tissue Eng. Part A 2011, 17, 631–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xue, Y.; Jia, B.; Bai, Y.; Zuo, Y.; Wang, S.; Zhao, Y.; Yang, W.; Tang, H. The preparation of hyaluronic acid grafted pullulan polymers and their use in the formation of novel biocompatible wound healing film. Carbohydr. Polym. 2018, 188, 92–100. [Google Scholar] [CrossRef]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- Magdassi, S.; Touitou, E. (Eds.) Novel Cosmetic Delivery Systems; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- Ogai, I.J.; Nep, E.U.; Audu-Peter, J.D. Advanced in Natural Polymers as Pharmaceutical Excipients. Pharm. Anal. Acta 2011, 3, 146. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, A.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-Hyaluronan Nanoparticles to Deliver Anti-Aging Active Ingredients Through the Skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef] [Green Version]
- Yildizimer, L.; Thanh, N.T.K.; Seifalian, A.M. Skin regeneration Scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 12. [Google Scholar] [CrossRef]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymerjc Plant-derived Excipients in Drug Delivery. Molecules 2009, 14, 2602–2629. [Google Scholar] [CrossRef]
- Loh, P.Y. Beauty and Personal Care Trends, In Cosmetics Asia, Euromonotor International, Bangkok. 12 February 2014. Available online: https://www.euromonitor.com/beauty-and-personal-care (accessed on 3 October 2019).
- Mordor Intelligence: Beauty and Personal Care Products Market-Growth, Trends, Forecast (2019–2024); Industry Report; Sachibouli: Hyderabad, India, 2019; Available online: https://www.mordorintelligence.com/ (accessed on 6 October 2019).
- Loh, P.Y. Global Trends in Beauty and Personal Care: Opportunities for Future, Euromonitor International. 2014. Available online: https://www.slideshare.net/Euromonitor/global-trends-in-beauty-and-personal-care (accessed on 31 January 2020).
- Loh, P.Y. Global Trends in Anti-Agers, Euromonitor International. 2014. Available online: https://www.slideshare.net/Euromonitor/global-trends-in-antiagers (accessed on 31 January 2020).
- Kim, B.S.; Mooney, D.Y. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Gigante, V.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rubber Toughening of Polylactic Acid (PLA) with Poly(butylene adipate-co-terephthalate) (PBAT): Mechanical Properties, Fracture Mechanics and Analysis of Ductile-to-Brittle Behavior while Varying Temperature and Test Speed. Eur. Polym. J. 2019, 115, 125–137. [Google Scholar] [CrossRef]
- Scott, C.R. Naturals in Cosmetic Science: The Bigger Picture. Pers. Care Eur. 2019, 13, 55–56. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Save Food: Global Food Losses and Food Waste; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011; Available online: http://www.fao.org./save-food/resources/keyfindings/en/ (accessed on 3 October 2019).
- FAO. Food Wastage Footprint. Impact on Natural Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Available online: http://www.fao.org/nt/sustainability (accessed on 3 October 2019).
- Morganti, P.; Morganti, G.; Chen, H.D.; Gagliardini, A. Beauty Mask: Market and Environment. J. Clin. Cosmet. Dermatol. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Israilides, C.; Smith, A.; Scanlon, B.; Barnett, C. Pullulan from Agro-Industrial Wastes. Biotechnol. Genet. Eng. Rev. 1999, 16, 309–324. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coltelli, M.-B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. J. Funct. Biomater. 2020, 11, 20. https://doi.org/10.3390/jfb11010020
Coltelli M-B, Danti S, De Clerck K, Lazzeri A, Morganti P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. Journal of Functional Biomaterials. 2020; 11(1):20. https://doi.org/10.3390/jfb11010020
Chicago/Turabian StyleColtelli, Maria-Beatrice, Serena Danti, Karen De Clerck, Andrea Lazzeri, and Pierfrancesco Morganti. 2020. "Pullulan for Advanced Sustainable Body- and Skin-Contact Applications" Journal of Functional Biomaterials 11, no. 1: 20. https://doi.org/10.3390/jfb11010020