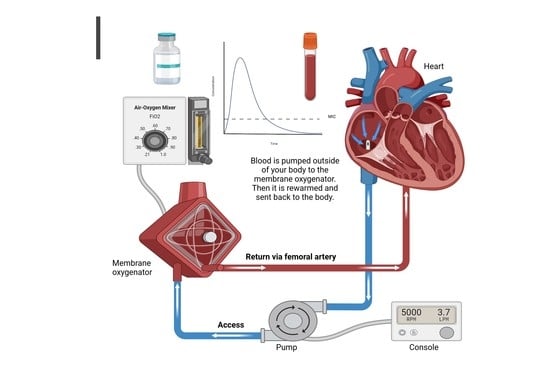
Sustaining Life versus Altering Life-Saving Drugs: Insights to Explain the Paradoxical Effect of Extracorporeal Membrane Oxygenation on Drugs
Abstract
:1. Introduction
2. ECMO Modalities
2.1. VA-ECMO
2.2. VV-ECMO
2.3. General Effects of ECMO on Drugs
2.4. Alterations in Drug PK Profiles during ECMO
2.4.1. Circuit and Drug Factors
Drug Sequestration
Circuit Priming
Circuit Age
Patient Factors
3. Management of Drug Treatments and Outcomes in Patients on ECMO
3.1. Anticoagulant Drug Monitoring and Outcomes in Patients on ECMO
3.1.1. Unfractionated Heparin UFH
3.1.2. Direct Thrombin Inhibitors
3.1.3. Anticoagulation in ECMO and CRRT Patients
3.1.4. Anticoagulant-Free ECMO Setting
3.1.5. Antiplatelet Therapy
| Study Type | ECLS Modality | Number of Patients | Anticoagulation Approach | Loading Dose | Initial Maintenance Dose | Adjustment to Dosing | Anticoagulation Target |
|---|---|---|---|---|---|---|---|
| UFH | |||||||
| Multi-center randomized control trial [45] | VV-ECMO | 42 | Continuous UFH | Bolus 70 or 50 units/kg | 18 units/kg/h | 12 h after first infusion, heparin was titrated to reach: (a) TEG target (b) aPPT target. |
|
| Retrospective chart review [46] | VV-ECMO or VA-ECMO | 123 | Continuous UFH | NA | 7 units/kg/h | (a) Supratherapeutic aPTT or TEG: heparin infusion reduced by 100 units/h. (b) Subtherapeutic, aPTT and TEG: heparin infusion increased by 100 units/h. (c) If one value is subtherapeutic and the other therapeutic, the patient’s anti-Xa level is measured. | aPTT: 60–80 s (1.5–2 × baseline) TEG: 2–4 × baseline anti-Xa level of 0.3–0.7 units/mL |
| Retrospective chart review [47] | VV-ECMO or VA-ECMO | 55 | UFH sparing; interrupted vs. continuous | Bolus at 100 units/kg | 10 units/kg/h or no heparin (≥3 d). | Titrate to ACT target | ACT: 170–230 |
| DTIs | |||||||
| Retrospective chart Review [53] | VV-ECMO or VA-ECMO | 9 | Argatroban | None | First patient 2 μg/kg/min. Subsequent patients 2 μg/kg/min | Titrate to aPTT target | aPTT: 50–60 s |
| Retrospective chart review [52] | VV-ECMO or VA-ECMO | 10 | Argatroban | None | Average initial argatroban dose was 0.175 μg/kg/min | Titrate by 0.05–0.1 μg/kg/min. | aPTT 55–75 s ACT 150–180 s |
| Case series [57] | VV-ECMO or VA-ECMO | 5 | Argatroban | None | 0.2–2 μg/kg/min | Titrated infusions using incremental changes of 0.05 μg/kg/min every hour to maintain ACTs target. | ACT: 210–230 s |
| Retrospective cohort study [61] | VV-ECMO or VA-ECMO | 13 | Bivalirudin | No bolus | Continuous infusion 0.03 to 0.05 mg/kg/h Halved starting dose in patients with reduced creatinine clearance. | Adjusted according to the ACT, aPTT, and r time values. |
|
| Retrospective cohort study [66] | VV-ECMO or VA-ECMO | 10 | Bivalirudin | No bolus | Continuous infusion of bivalirudin 0.025 mg/kg/h. | When needed, bivalirudin or heparin infusion was increased or decreased step by step, never exceeding 15% of the previous dosage. If a supramaximal aPTT value was recorded, drug infusion was discontinued for 2 h and then started again at a 15% lower dose. | aPTT: 45 to 60 s. |
| Retrospective study [67] | VV-ECMO or VA-ECMO | 44 | Bivalirudin | No bolus | Continuous infusion of bivalirudin 0.04 mg/kg/h | Adjusted per aPTT goal. | Low-intensity (45–65 s) or high-intensity (60–80 s) |
| Single-center, retrospective, observational analysis [63] | VV-ECMO or VA-ECMO | 14 | Bivalirudin | No bolus (except for one patient, who received 0.2 mg/kg) | 0.02 to 0.26 mg/kg/h | Adjusted based on aPTT. | aPTT: 1.5–2.5 times the patient’s baseline. 1.5–2.0 × baseline for those with bleeding concerns |
| ECMO patients on CRRT | |||||||
| Single-center, retrospective, observational analysis [63] | VV-ECMO or VA-ECMO | 4 | Bivalirudin for patients on both ECLS and CRRT | No bolus | Median dose of 0.21 mg/kg/h | Adjusted to maintain: aPPT target The median maximum rate patients were titrated to = 0.36 mg/kg/h Individual patients required between 75% and 125% bivalirudin rate increases over the first 48–120 h on CRRT. | aPTT: 1.5–2.5 times the patient’s baseline aPTT. 1.5–2.0 × baseline for those with bleeding concerns |
| Retrospective chart review [84] | VV-ECMO and simultaneous CRRT (HD mode) | 22 | UFH and RCA | 1—UFH: IV bolus of UFH (50–70 unit/kg) 2—RCA: no bolus | 1—UFH continuous heparin infusion (starting at 18 IU/kg/h) 2—RCA: NA | 1-a—UFH adjusted during the first 12 h to reach ACT target. 1-b—Heparin infusion is titrated to a target aPTT ratio 2—RCA:NA | ACT: 170–200 s For first 12 h, then aPTT ratio: 1.5 × baseline |
| Retrospective, single-center study [83] | VV-ECMO and simultaneous CRRT | 29 | ACDA with or without heparin | Heparin: not mentioned ACD: No bolus | ACDA: at a fixed rate of 240 mL/h | not specified | not specified |
3.1.6. Management of Antimicrobial Drugs in Patients on ECMO
4. Aminoglycoside Antibiotics
4.1. Amikacin
4.1.1. Beta-Lactam (Carbapenem) Antibiotics
Imipenem
Meropenem
Piperacillin/Tazobactam
4.1.2. Glycopeptide Antibiotics
Vancomycin
4.1.3. Macrolide Antibiotics
Azithromycin
4.1.4. Neuraminidase Inhibitors
Oseltamivir
4.1.5. Oxazolidinone Antibiotics
Linezolid
4.1.6. Antifungal Agents
4.1.7. Antituberculosis Agents
4.2. Management of Sedation and Analgesia in Patients on ECMO
4.2.1. Sedation
| Antimicrobial/Study Type | ECLS Modality | Number of Patients | Reported PK Parameter | Dose | Study-Specific Recommendations |
|---|---|---|---|---|---|
| Aminoglycoside antibiotics | |||||
| Amikacin Case–control study [103] | VV or VA-ECMO | 46 | Cmax (mg/L): 71.7 (58.9–79.7) Cmax < 60 mg/L in 26% Cmax > 80 mg/L in 24% AUC (mg.h/mL): 973 (799–1193) Cmin (mg/L): 8.5 (3.0–15.4) | 15–20 mg/kg doses, interval by TDM. |
|
| Beta-lactam (carbapenem) antibiotics | |||||
| Imipenem Case Series [104] Case Series [105] | VV-ECMO VV or VA-ECMO | 2 10 | MIC mg/L: 0.125 and 0.25. Vd (L): 13.98 CL (L/h): 9.78 | 1 g q6 h. 0.5 g q6 h |
|
| Meropenem 1-Case–control study [95] 2-Matched cohort study [106] | VV or VA-ECMO (9 on CRRT) VV-ECMO or VA-ECMO (3 on CRRT) | 26 14 | Vd (L/kg): 0.46 (0.26–0.92) t ½ (h): 3.0 (2.1–4.8) CL (mL/min): 125 (63–198) Vd (L/kg): 29.7 ± 19.2 ve CL (mL/min): 17.4 ± 14.8 L/h | At Cr CL of: a: >80 mL/min: 1 g q8 h b: 51–80 mL/min: 1 g q12 h c: 10–50 mL/min: 0.5 g q12 h d: <10 mL/min: 0.5 g daily -CRRT: 1 g q8 h 1 g (IV) bolus and 1 g IV q8 h |
|
| Piperacillin/tazobactam: 1-Case control study [95] Prospective, open-labeled, multicenter PK study [107] | VV-ECMO or VA-ECMO (9 on CRRT) VV and VA-ECMO (14 on RRT) | 14 27 | Vd (L/kg): 0.33 (0.26–0.46) t1/2 (h): 2.0 (1.1–4.2) CL (mL/min): 156 (91–213) Vd (L/kg): 0.51 CL (L/h): 12.02 | At Cr CL of a: >80 mL/min: 4.5 g q6 h b: 51–80 mL/min: 4.5 g q6 h c: 10–50 mL/min: 4.5 g q6 h d <10 mL/min: 4.5 g q6 h -CRRT: 4.5 g q6 h 4.5 g LD, then 4.5 g q6–8 h |
|
| Glycopeptide antibiotics | |||||
| Vancomycin Retrospective study [109] Prospective, matched cohort, single center, pharmacokinetic study [110] | 1-VV and VA-ECMO | 20 11 | K (h−1): 0.12 ± 0.04 CL (L/h): 4.62 Vd (L/kg): 0.65 K (h−1): 0.088 ((0.055)) Vd (L/kg): 0.84 (0.24) | Total daily dose: 32.54 mg/kg q2.10 ± 0.72/day. Initial dose: 15–25 mg/kg Maintenance dose: calculated to achieve to achieve trough levels within 10–20 mg/L. |
|
| Macrolide antibiotics | |||||
| Azithromycin [114] | VV-ECMO | 3 | Cmax (mg/L): 4.0 ± 0.5. C24 (mg/L): 0.22 ± 0.1 AUC0–24 (mg-h/L): 9.8 ± 2.6 CL (mL/min/kg): 8.0 ± 4.9 Vd (L/kg): 19.8 ± 7.6 | -IV infusion of 500 mg q24 h |
|
| Neuraminidase inhibitors | |||||
| Oseltamivir [115] Single-center, prospective, open-label, population PK study [117] | VV-ECMO (4 on CVVHF) VV-ECMO (3 on concomitant CVVHF) | 14 7 | CL (L/h): 15.8 (4.8–36.6) Vd (L): 179 (61–436) AUC (ng/hour/mL): 4346 (644–13,660) Cmax (ng/mL): 509 (54–1277) -Patients with preserved renal function: Cmax (ng/mL): 1029 ± 478 AUC (mcg/h/mL): 9.00 ± 4.52 -Patients on ECMO and CVVHF: 4- to 5-fold higher Cmax and AUC | 75 mg twice daily 75 or 150 mg twice daily |
|
| Oxazolidinone antibiotics | |||||
| Linezolid case-series study [118] | NA | 3 | Cmax (mg/L): 15.67, 18.51 and 15.61. Cmin (mg/L): 4.25, 0.47 and 0.43. AUC0–24 (mg h/L): 212.58, 165.65 and 100.59. CL (L/h): 5.65 7.24 13.35 Vd (L): 49.7, 17.6 and 46.77 t1/2 (h): 6.10, 1.68 and 2.20 | Infusion: 600 mg q12 h | NA
|
| Antifungal agents | |||||
| Voriconazole and caspofungin Case series study [120] | VV-ECMO | 2 | Caspofungin: -Mean trough (μg/mL): 3.73 -mean peak (μg/mL): 11.95 -t1/2 (h): 13.60 -Vd (L): 8.22 -CL (mL/min): 6.90 Variconazole: -Mean trough (μg/mL): 9.65 -Mean peak (μg/mL): 13.91 -t1/2 (h): 21 -Vd (L): 1.38 -CL (mL/min): 49.33 | Caspofungin: loading dose: 70 mg/day maintenance dose: 70 mg once daily. Variconazole: IV loading dose: 400 mg twice daily maintenance dose: 280 mg twice daily. |
|
| Antituberculosis agents | |||||
| Ethambutol and rifampicin Case report [122] | VV-ECMO/extended dialysis | 1 | Dialyzer clearance: Ethambutol: Whole blood: 1 mL/min (range 51–131 mL/min) Plasma: 95 mL/min Rifampicin: Whole blood: Between 53 and 77 mL/min Plasma: Between 39 and 53 mL/min. |
|
|
| Ethambutol/rifampicin and Pyrazinamide [123] | VV-ECMO | 1 | Rifampicin: Serum Cmax (μg/mL): 8–24 Serum Tmax (h): 0.75–2/ Ethambutol: Serum Cmax (μg/mL): 2–6 Serum Tmax (h): 2–3 Pyrazinaminde: Serum Cmax (μg/mL): 20–50 Serum Tmax (h): 1–2 | Rifampicin: 750 to 1200 mg/day Ethambutol: 1200 to 1600 mg/day Pyrazinaminde: IV: 1200 to 1600 |
|
4.2.2. Analgesia
Parenteral Opioid Analgesia
4.2.3. Nonopioid Analgesia
| Sedative/Study Type | ECLS Modality | Number of Patients | Dose | Recommendations |
|---|---|---|---|---|
| Midazolam -Retrospective chart review [128] | VV or VA-ECMO and RRT | 29 |
|
|
| -Retrospective comparative cohort analysis [129] -Benzodiazepines/prospective, observational study [137] | VV or VA-ECMO (11 on RRT) VV or VA-ECMO | 34 32 |
|
|
| -Opioids/prospective, observational study [137] -Hydromorphone and fentanyl/single-center retrospective observational study [139] | VV or VA-ECMO VV or VA-ECMO | 32 148 |
|
|
| Methadone/case series study [148] | VV-ECMO | 2 | Case 1: 30 mg intravenous 4 times a day/40 mg by mouth 4 times a day Case 2: 10 mg intravenous 3 times a day/40 mg by mouth 3 times a day) | Effectiveness was demonstrated by decreasing other opiates and sedatives without the need for dose escalation in this population. |
| Ketamine -Case series [149] -A randomized controlled trial [151] | VV or VA-ECMO 2-VV-ECMO | 26 10 |
|
|
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741.e9–741.e18. [Google Scholar] [CrossRef]
- Al-Fares, A.; Pettenuzzo, T.; Del Sorbo, L. Extracorporeal life support and systemic inflammation. Intensive Care Med. Exp. 2019, 7 (Suppl. S1), 46. [Google Scholar] [CrossRef]
- Datzmann, T.; Träger, K. Extracorporeal membrane oxygenation and cytokine adsorption. J. Thorac. Dis. 2018, 10 (Suppl. S5), S653–S660. [Google Scholar] [CrossRef]
- The 3rd Chinese Society of Extracorporeal Life Support Annual Meeting. Available online: https://csecls2019.medmeeting.org/8735?lang=cn (accessed on 29 March 2023).
- Extracorporeal Life Support Organization (ELSO) Report in 2018. Available online: https://www.elso.org/ (accessed on 29 March 2023).
- Ratnani, I.; Tuazon, D.; Zainab, A.; Uddin, F. The Role and Impact of Extracorporeal Membrane Oxygenation in Critical Care. Methodist DeBakey Cardiovasc. J. 2018, 14, 110–119. [Google Scholar] [CrossRef]
- Napp, L.C.; Kühn, C.; Hoeper, M.M.; Vogel-Claussen, J.; Haverich, A.; Schäfer, A.; Bauersachs, J. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin. Res. Cardiol. 2016, 105, 283–296. [Google Scholar] [CrossRef]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef] [PubMed]
- Keebler, M.E.; Haddad, E.V.; Choi, C.W.; McGrane, S.; Zalawadiya, S.; Schlendorf, K.H.; Brinkley, D.M.; Danter, M.R.; Wigger, M.; Menachem, J.N.; et al. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail. 2018, 6, 503–516. [Google Scholar] [CrossRef]
- Jayaraman, A.L.; Cormican, D.; Shah, P.; Ramakrishna, H. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: Techniques, limitations, and special considerations. Ann. Card. Anaesth. 2017, 20, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Mazzeffi, M.; Galvagno, S.; Menaker, J. VV ECMO Cannulation: Should I Stay or Should I Go? J. Cardiothorac. Vasc. Anesth. 2019, 33, 1871–1872. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Goffi, A.; Goligher, E.; Fan, E.; Slutsky, A.S. Setting mechanical ventilation in ARDS patients during VV-ECMO: Where are we? Minerva Anestesiol. 2015, 81, 1369–1376. [Google Scholar]
- Shekar, K.; Roberts, J.A.; Smith, M.T.; Fung, Y.L.; Fraser, J.F. The ECMO PK Project: An incremental research approach to advance understanding of the pharmacokinetic alterations and improve patient outcomes during extracorporeal membrane oxygenation. BMC Anesthesiol. 2013, 13, 7. [Google Scholar] [CrossRef]
- Preston, T.J.; Ratliff, T.M.; Gomez, D.; Olshove, V.E., Jr.; Nicol, K.K.; Sargel, C.L.; Chicoine, L.G. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J. ExtraCorporeal Technol. 2010, 42, 199–202. [Google Scholar]
- Preston, T.J.; Hodge, A.B.; Riley, J.B.; Leib-Sargel, C.; Nicol, K.K. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit. J. ExtraCorporeal Technol. 2007, 39, 234–237. [Google Scholar]
- Wu, F.; Li, M.; Zhang, Z.; Shang, J.; Guo, Y.; Li, Y. Sedation, Analgesia, and Muscle Relaxation During VV-ECMO Therapy in Patients with Severe Acute Respiratory Syndrome Coronavirus Type 2 (SARS-CoV-2): A Single-Center, Retrospective, Observational Study. Front. Med. (Lausanne) 2021, 8, 762740. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.W.; Anderson, B.J.; Simons, S.H.; Uges, D.R.; Tibboel, D. Morphine pharmacokinetics during venoarterial extracorporeal membrane oxygenation in neonates. Intensiv. Care Med. 2005, 31, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Raffaeli, G.; Cavallaro, G.; Allegaert, K.; Koch, B.C.P.; Mosca, F.; Tibboel, D.; Wildschut, E.D. Sequestration of Voriconazole and Vancomycin into Contemporary Extracorporeal Membrane Oxygenation Circuits: An in vitro Study. Front. Pediatr. 2020, 8, 468. [Google Scholar] [CrossRef]
- Poole, S.K.; Poole, C.F. Separation methods for estimating octanol-water partition coefficients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 797, 3–19. [Google Scholar] [CrossRef]
- Eyler, R.F.; Heung, M.; Pleva, M.; Sowinski, K.M.; Park, P.K.; Napolitano, L.M.; Mueller, B.A. Pharmacokinetics of oseltamivir and oseltamivir carboxylate in critically ill patients receiving continuous venovenous hemodialysis and/or extracorporeal membrane oxygenation. Pharmacotherapy 2012, 32, 1061–1069. [Google Scholar] [CrossRef]
- Watt, K.M.; Benjamin, D.K., Jr.; Cheifetz, I.M.; Moorthy, G.; Wade, K.C.; Smith, P.B.; Brouwer, K.L.; Capparelli, E.V.; Cohen-Wolkowiez, M. Pharmacokinetics and safety of fluconazole in young infants supported with extracorporeal membrane oxygenation. Pediatr. Infect. Dis. J. 2012, 31, 1042–1047. [Google Scholar] [CrossRef]
- Wagner, D.; Pasko, D.; Phillips, K.; Waldvogel, J.; Annich, G. In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation. Perfusion 2013, 28, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.A.; Sieg, A.C. Evaluation of Altered Drug Pharmacokinetics in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation. Pharmacotherapy 2017, 37, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Heith, C.S.; Hansen, L.A.; Bakken, R.M.; Ritter, S.L.; Long, B.R.; Hume, J.R.; Zhang, L.; Amundsen, D.B.; Steiner, M.E.; Fischer, G.A. Effects of an Ex Vivo Pediatric Extracorporeal Membrane Oxygenation Circuit on the Sequestration of Mycophenolate Mofetil, Tacrolimus, Hydromorphone, and Fentanyl. J. Pediatr. Pharmacol. Ther. 2019, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Extracorporeal Life Support Registry Report. Available online: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx (accessed on 15 August 2016).
- Boucher, B.A.; Wood, G.C.; Swanson, J.M. Pharmacokinetic changes in critical illness. Crit. Care Clin. 2006, 22, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851; quiz 859. [Google Scholar] [CrossRef] [PubMed]
- Power, B.M.; Forbes, A.M.; van Heerden, P.V.; Ilett, K.F. Pharmacokinetics of drugs used in critically ill adults. Clin. Pharmacokinet. 1998, 34, 25–56. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Heiden, A.M.; Beutel, G.; Gottlieb, J.; Wiesner, O.; Hafer, C.; Hadem, J.; Reising, A.; Haverich, A.; Kühn, C.; et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol. Dial. Transplant. 2013, 28, 86–90. [Google Scholar] [CrossRef]
- Many, M.; Soroff, H.S.; Birtwell, W.C.; Giron, F.; Wise, H.; Deterling, R.A., Jr. The physiologic role of pulsatile and nonpulsatile blood flow. II. Effects on renal function. Arch. Surg. 1967, 95, 762–767. [Google Scholar] [CrossRef]
- Bakhtiary, F.; Keller, H.; Dogan, S.; Dzemali, O.; Oezaslan, F.; Meininger, D.; Ackermann, H.; Zwissler, B.; Kleine, P.; Moritz, A. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: Clinical experiences in 45 adult patients. J. Thorac. Cardiovasc. Surg. 2008, 135, 382–388. [Google Scholar] [CrossRef]
- Beiras-Fernandez, A.; Deutsch, M.A.; Kainzinger, S.; Kaczmarek, I.; Sodian, R.; Ueberfuhr, P.; Meiser, B.; Schmoeckel, M.; Reichart, B.; Brenner, P. Extracorporeal membrane oxygenation in 108 patients with low cardiac output—A single-center experience. Int. J. Artif. Organs 2011, 34, 365–373. [Google Scholar] [CrossRef]
- Trudzinski, F.C.; Minko, P.; Rapp, D.; Fähndrich, S.; Haake, H.; Haab, M.; Bohle, R.M.; Flaig, M.; Kaestner, F.; Bals, R.; et al. Runtime and aPTT predict venous thrombosis and thromboembolism in patients on extracorporeal membrane oxygenation: A retrospective analysis. Ann. Intensive Care 2016, 6, 66. [Google Scholar] [CrossRef]
- Rastan, A.J.; Lachmann, N.; Walther, T.; Doll, N.; Gradistanac, T.; Gommert, J.F.; Lehmann, S.; Wittekind, C.; Mohr, F.W. Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (ECMO). Int. J. Artif. Organs 2006, 29, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Lequier, L.; Annich, G.; Al-Ibrahim, O.; Bembea, M.; Brodie, D.; Brogan, T. Elso Anticoagulation Guidelines; Extracorporeal Life Support Organization: Ann Arbor, MI, USA, 2014. [Google Scholar]
- Koster, A.; Ljajikj, E.; Faraoni, D. Traditional and non-traditional anticoagulation management during extracorporeal membrane oxygenation. Ann. Cardiothorac. Surg. 2019, 8, 129–136. [Google Scholar] [CrossRef]
- Olson, J.D.; Arkin, C.F.; Brandt, J.T.; Cunningham, M.T.; Giles, A.; Koepke, J.A.; Witte, D.L. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: Laboratory monitoring of unfractionated heparin therapy. Arch. Pathol. Lab. Med. 1998, 122, 782–798. [Google Scholar]
- Bates, S.M.; Weitz, J.I.; Johnston, M.; Hirsh, J.; Ginsberg, J.S. Use of a fixed activated partial thromboplastin time ratio to establish a therapeutic range for unfractionated heparin. Arch. Intern. Med. 2001, 161, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Dzik, W.H. The James Blundell Award Lecture 2006: Transfusion and the treatment of haemorrhage: Past, present and future. Transfus. Med. 2007, 17, 367–374. [Google Scholar] [CrossRef]
- Hoffman, M.; Monroe, D.M. Coagulation 2006: A modern view of hemostasis. Hematol. Oncol. Clin. North Am. 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Esper, S.A.; Levy, J.H.; Waters, J.H.; Welsby, I.J. Extracorporeal membrane oxygenation in the adult: A review of anticoagulation monitoring and transfusion. Anesth. Analg. 2014, 118, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C. Anticoagulation and coagulation management for ECMO. Semin. Cardiothorac. Vasc. Anesth. 2009, 13, 154–175. [Google Scholar] [CrossRef]
- Sulkowski, J.P.; Preston, T.J.; Cooper, J.N.; Duffy, V.L.; Deans, K.J.; Chicoine, L.G.; Minneci, P.C. Comparison of routine laboratory measures of heparin anticoagulation for neonates on extracorporeal membrane oxygenation. J. ExtraCorporeal Technol. 2014, 46, 69–76. [Google Scholar]
- Lv, X.; Deng, M.; Wang, L.; Dong, Y.; Chen, L.; Dai, X. Low vs standardized dose anticoagulation regimens for extracorporeal membrane oxygenation: A meta-analysis. PLoS ONE 2021, 16, e0249854. [Google Scholar] [CrossRef]
- Panigada, M.; EIapichino, G.; Brioni, M.; Panarello, G.; Protti, A.; Grasselli, G.; Occhipinti, G.; Novembrino, C.; Consonni, D.; Arcadipane, A.; et al. Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: A safety and feasibility pilot study. Ann. Intensive Care 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Colman, E.; Yin, E.B.; Laine, G.; Chatterjee, S.; Saatee, S.; Herlihy, J.P.; Reyes, M.A.; Bracey, A.W. Evaluation of a heparin monitoring protocol for extracorporeal membrane oxygenation and review of the literature. J. Thorac. Dis. 2019, 11, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Cho, D.Y.; Sohn, D.S.; Lee, W.S.; Won, H.; Lee, D.H.; Kang, H.; Hong, J. Is Stopping Heparin Safe in Patients on Extracorporeal Membrane Oxygenation Treatment? ASAIO J. 2017, 63, 32–36. [Google Scholar] [CrossRef]
- Lee, C.J.; Ansell, J.E. Direct thrombin inhibitors. Br. J. Clin. Pharmacol. 2011, 72, 581–592, Erratum in Br. J. Clin. Pharmacol. 2011, 72, 718. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Yonekawa, K.E.; Nakagawa, P.; Nugent, D.J. Argatroban as an alternative to heparin in extracorporeal membrane oxygenation circuits. Perfusion 2004, 19, 283–288. [Google Scholar] [CrossRef]
- Bates, S.M.; Weitz, J.I. The mechanism of action of thrombin inhibitors. J. Invasive Cardiol. 2000, 12 (Suppl. SF), 27F–32F. [Google Scholar]
- Di Nisio, M.; Middeldorp, S.; Büller, H.R. Direct thrombin inhibitors. N. Engl. J. Med. 2005, 353, 1028–1040, Erratum in N. Engl. J. Med. 2005, 353, 2827. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.; Yang, J.H.; Sung, K.; Suh, G.Y.; Chung, C.R.; Yang, J.H.; Cho, Y.H. Use of argatroban for extracorporeal life support in patients with nonheparin-induced thrombocytopenia: Analysis of 10 consecutive patients. Medicine (Baltimore) 2018, 97, e13235. [Google Scholar] [CrossRef] [PubMed]
- Beiderlinden, M.; Treschan, T.; Görlinger, K.; Peters, J. Argatroban in extracorporeal membrane oxygenation. Artif. Organs 2007, 31, 461–465. [Google Scholar] [CrossRef]
- Geli, J.; Capoccia, M.; Maybauer, D.M.; Maybauer, M.O. Argatroban Anticoagulation for Adult Extracorporeal Membrane Oxygenation: A Systematic Review. J. Intensive Care Med. 2022, 37, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Greinacher, A.; Koster, A.; Lincoff, A.M. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133 (Suppl. S6), 340S–380S, Erratum in Chest 2011, 139, 1261. [Google Scholar] [CrossRef]
- Linkins, L.A.; Dans, A.L.; Moores, L.K.; Bona, R.; Davidson, B.L.; Schulman, S.; Crowther, M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e495S–e530S, Erratum in Chest 2015, 148, 1529. [Google Scholar] [CrossRef]
- Cornell, T.; Wyrick, P.; Fleming, G.; Pasko, D.; Han, Y.; Custer, J.; Haft, J.; Annich, G. A case series describing the use of argatroban in patients on extracorporeal circulation. ASAIO J. 2007, 53, 460–463. [Google Scholar] [CrossRef]
- Alatri, A.; Armstrong, A.E.; Greinacher, A.; Koster, A.; Kozek-Langenecker, S.A.; Lancé, M.D.; Link, A.; Nielsen, J.D.; Sandset, P.M.; Spanjersberg, A.J.; et al. Results of a consensus meeting on the use of argatroban in patients with heparin-induced thrombocytopenia requiring antithrombotic therapy—A European Perspective. Thromb. Res. 2012, 129, 426–433. [Google Scholar] [CrossRef]
- Kelton, J.G.; Arnold, D.M.; Bates, S.M. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N. Engl. J. Med. 2013, 368, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.; Valgimigli, M.; Sunnåker, M.; Vranckx, P.; Frigoli, E.; Leonardi, S.; Spirito, A.; Gragnano, F.; Manavifar, N.; Galea, R.; et al. Choice of access site and type of anticoagulant in acute coronary syndromes with advanced Killip class or out-of-hospital cardiac arrest. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 893–901, English, Spanish. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Kandil, H.; Isgrò, G.; Carlucci, C.; Baryshnikova, E.; Pistuddi, V.; Surgical and Clinical Outcome Research Group. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit. Care 2011, 15, R275. [Google Scholar] [CrossRef]
- Kaseer, H.; Soto-Arenall, M.; Sanghavi, D.; Moss, J.; Ratzlaff, R.; Pham, S.; Guru, P. Heparin vs bivalirudin anticoagulation for extracorporeal membrane oxygenation. J. Card. Surg. 2020, 35, 779–786. [Google Scholar] [CrossRef]
- Walker, E.A.; Roberts, A.J.; Louie, E.L.; Dager, W.E. Bivalirudin Dosing Requirements in Adult Patients on Extracorporeal Life Support with or Without Continuous Renal Replacement Therapy. ASAIO J. 2019, 65, 134–138. [Google Scholar] [CrossRef]
- Pappalardo, F.; Maj, G.; Scandroglio, A.; Sampietro, F.; Zangrillo, A.; Koster, A. Bioline heparin-coated ECMO with bivalirudin anticoagulation in a patient with acute heparin-induced thrombocytopenia: The immune reaction appeared to continue unabated. Perfusion 2009, 24, 135–137. [Google Scholar] [CrossRef]
- Chen, E.; Clarke, N.; Huffman, L.; Peltz, M. Transplantation in a patient on extracorporeal membrane oxygenation with infective endocarditis, pericarditis and heparin-induced thrombocytopenia. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 462–463. [Google Scholar] [CrossRef]
- Pieri, M.; Agracheva, N.; Bonaveglio, E.; Greco, T.; De Bonis, M.; Covello, R.D.; Zangrillo, A.; Pappalardo, F. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: A case-control study. J. Cardiothorac. Vasc. Anesth. 2013, 27, 30–34. [Google Scholar] [CrossRef]
- Berei, T.J.; Lillyblad, M.P.; Wilson, K.J.; Garberich, R.F.; Hryniewicz, K.M. Evaluation of Systemic Heparin Versus Bivalirudin in Adult Patients Supported by Extracorporeal Membrane Oxygenation. ASAIO J. 2018, 64, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Natt, B.; Hypes, C.; Basken, R.; Malo, J.; Kazui, T.; Mosier, J. Suspected Heparin-Induced Thrombocytopenia in Patients Receiving Extracorporeal Membrane Oxygenation. J. ExtraCorporeal Technol. 2017, 49, 54–58. [Google Scholar]
- Ljajikj, E.; Zittermann, A.; Morshuis, M.; Börgermann, J.; Ruiz-Cano, M.; Schoenbrodt, M.; Gummert, J.; Koster, A. Bivalirudin anticoagulation for left ventricular assist device implantation on an extracorporeal life support system in patients with heparin-induced thrombocytopenia antibodies. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 898–904, Erratum in Interact. Cardiovasc. Thorac. Surg. 2017, 25, 675. [Google Scholar] [CrossRef]
- Koster, A.; Niedermeyer, J.; Gummert, J.; Renner, A. Low dose bivalirudin anticoagulation for lung transplantation with extracorporeal membrane oxygenation in a patient with acute heparin-induced thrombocytopenia. Eur. J. Cardiothorac. Surg. 2017, 51, 1009–1011. [Google Scholar] [CrossRef]
- Klompas, A.M.; Albright, R.C.; Maltais, S.; Demirci, O. Acute renal failure due to bilateral renal vein thromboses: A rare complication of heparin-induced thrombocytopenia. Ann. Card. Anaesth. 2019, 22, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Van Sint Jan, N.; Díaz, R.; Fajardo, C.; Agliatti, R.; Palavecino, M.; Hasbún, P.; Regueira, T. Factibilidad del uso de bivalirudina como anticoagulante en soporte vital extracorpóreo [Experience with anticoagulation with bivalirudin during extracorporeal membrane oxygenation]. Rev. Med. Chil. 2017, 145, 710–715. [Google Scholar] [CrossRef]
- Abdelbary, A.; Khaled, M.; Sami, W.; Said, A.; Yosri, M.; Abuelwafa, M.; Saad, M.; Tawfik, H.; Zoghbi, I.; Abouelgheit, M.; et al. Initial Egyptian ECMO experience. Egypt. J. Crit. Care Med. 2016, 4, 25–32. [Google Scholar] [CrossRef]
- Koster, A.; Weng, Y.; Böttcher, W.; Gromann, T.; Kuppe, H.; Hetzer, R. Successful use of bivalirudin as anticoagulant for ECMO in a patient with acute HIT. Ann. Thorac. Surg. 2007, 83, 1865–1867. [Google Scholar] [CrossRef]
- Pazhenkottil, A.P.; Rudiger, A.; Flammer, A.; Enseleit, F.; Jacobs, S.; Falk, V.; Ruschitzka, F.; Bettex, D. Left Main Artery Thrombus Complicating Heart Transplantation in a Patient with Heparin-Induced Thrombocytopenia. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1334–1336. [Google Scholar] [CrossRef]
- Zhong, H.; Zhu, M.L.; Yu, Y.T.; Li, W.; Xing, S.P.; Zhao, X.Y.; Wang, W.J.; Gu, Z.C.; Gao, Y. Management of Bivalirudin Anticoagulation Therapy for Extracorporeal Membrane Oxygenation in Heparin-Induced Thrombocytopenia: A Case Report and a Systematic Review. Front. Pharmacol. 2020, 11, 565013. [Google Scholar] [CrossRef] [PubMed]
- Skrupky, L.P.; Smith, J.R.; Deal, E.N.; Arnold, H.; Hollands, J.M.; Martinez, E.J.; Micek, S.T. Comparison of bivalirudin and argatroban for the management of heparin-induced thrombocytopenia. Pharmacotherapy 2010, 30, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- M’Pembele, R.; Roth, S.; Metzger, A.; Nucaro, A.; Stroda, A.; Polzin, A.; Hollmann, M.W.; Lurati Buse, G.; Huhn, R. Evaluation of clinical outcomes in patients treated with heparin or direct thrombin inhibitors during extracorporeal membrane oxygenation: A systematic review and meta-analysis. Thromb. J. 2022, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Berlioz, B.; Kaseer, H.S.; Sanghavi, D.K.; Guru, P.K. Bivalirudin resistance in a patient on veno-venous extracorporeal membrane oxygenation with a therapeutic response to argatroban. BMJ Case Rep. 2020, 13, e232262. [Google Scholar] [CrossRef]
- Streng, A.S.; Delnoij, T.S.R.; Mulder, M.M.G.; Sels, J.W.E.M.; Wetzels, R.J.H.; Verhezen, P.W.M.; Olie, R.H.; Kooman, J.P.; van Kuijk, S.M.J.; Brandts, L.; et al. Monitoring of Unfractionated Heparin in Severe COVID-19: An Observational Study of Patients on CRRT and ECMO. TH Open 2020, 4, e365–e375. [Google Scholar] [CrossRef] [PubMed]
- Schilder, L.; Nurmohamed, S.A.; Bosch, F.H.; Purmer, I.M.; den Boer, S.S.; Kleppe, C.G.; Vervloet, M.G.; Beishuizen, A.; Girbes, A.R.; Ter Wee, P.M.; et al. Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: A multi-center randomized clinical trial. Crit. Care 2014, 18, 472. [Google Scholar] [CrossRef]
- Stucker, F.; Ponte, B.; Tataw, J.; Martin, P.Y.; Wozniak, H.; Pugin, J.; Saudan, P. Efficacy and safety of citrate-based anticoagulation compared to heparin in patients with acute kidney injury requiring continuous renal replacement therapy: A randomized controlled trial. Crit. Care 2015, 19, 91. [Google Scholar] [CrossRef]
- Shum, H.P.; Kwan, A.M.; Chan, K.C.; Yan, W.W. The use of regional citrate anticoagulation continuous venovenous hemofiltration in extracorporeal membrane oxygenation. ASAIO J. 2014, 60, 413–418. [Google Scholar] [CrossRef]
- Giani, M.; Scaravilli, V.; Stefanini, F.; Valsecchi, G.; Rona, R.; Grasselli, G.; Bellani, G.; Pesenti, A.M.; Foti, G. Continuous Renal Replacement Therapy in Venovenous Extracorporeal Membrane Oxygenation: A Retrospective Study on Regional Citrate Anticoagulation. ASAIO J. 2020, 66, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; Mccarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J. 2021, 67, 290–296. [Google Scholar] [CrossRef]
- Tomasko, J.; Prasad, S.M.; Dell, D.O.; DeCamp, M.M.; Bharat, A. Therapeutic anticoagulation-free extracorporeal membrane oxygenation as a bridge to lung transplantation. J. Heart Lung Transplant. 2016, 35, 947–948. [Google Scholar] [CrossRef]
- Robba, C.; Ortu, A.; Bilotta, F.; Lombardo, A.; Sekhon, M.S.; Gallo, F.; Matta, B.F. Extracorporeal membrane oxygenation for adult respiratory distress syndrome in trauma patients: A case series and systematic literature review. J. Trauma Acute Care Surg. 2017, 82, 165–173. [Google Scholar] [CrossRef]
- Mangoush, O.; Purkayastha, S.; Haj-Yahia, S.; Kinross, J.; Hayward, M.; Bartolozzi, F.; Darzi, A.; Athanasiou, T. Heparin-bonded circuits versus nonheparin-bonded circuits: An evaluation of their effect on clinical outcomes. Eur. J. Cardiothorac. Surg. 2007, 31, 1058–1069. [Google Scholar] [CrossRef]
- Ranucci, M.; Balduini, A.; Ditta, A.; Boncilli, A.; Brozzi, S. A systematic review of biocompatible cardiopulmonary bypass circuits and clinical outcome. Ann. Thorac. Surg. 2009, 87, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.J.; Deakin, C.D.; Morley, P.T.; Callaway, C.W.; Kerber, R.E.; Kronick, S.L.; Lavonas, E.J.; Link, M.S.; Neumar, R.W.; Otto, C.W.; et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2010, 122 (Suppl. S2), S345–S421. [Google Scholar] [CrossRef]
- Bossaert, L.; O’Connor, R.E.; Arntz, H.R.; Brooks, S.C.; Diercks, D.; Feitosa-Filho, G.; Nolan, J.P.; Hoek, T.L.; Walters, D.L.; Wong, A.; et al. Part 9: Acute coronary syndromes: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2010, 81 (Suppl. S1), e175–e212. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Jüni, P.; et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Rev. Esp. Cardiol. (Engl. Ed.) 2015, 68, 144. [Google Scholar] [CrossRef]
- Staudacher, D.L.; Biever, P.M.; Benk, C.; Ahrens, I.; Bode, C.; Wengenmayer, T. Dual Antiplatelet Therapy (DAPT) versus No Antiplatelet Therapy and Incidence of Major Bleeding in Patients on Venoarterial Extracorporeal Membrane Oxygenation. PLoS ONE 2016, 11, e0159973. [Google Scholar] [CrossRef]
- Baldetti, L.; Nardelli, P.; Ajello, S.; Melisurgo, G.; Calabrò, M.G.; Pieri, M.; Scandroglio, A.M. Anti-thrombotic Therapy with Cangrelor and Bivalirudin in Venoarterial Extracorporeal Membrane Oxygenation Patients Undergoing Percutaneous Coronary Intervention: A Single-Center Experience. ASAIO J. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Donadello, K.; Antonucci, E.; Cristallini, S.; Roberts, J.A.; Beumier, M.; Scolletta, S.; Jacobs, F.; Rondelet, B.; de Backer, D.; Vincent, J.L.; et al. β-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int. J. Antimicrob. Agents 2015, 45, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Abdul-Aziz, M.H.; Roberts, J.A.; Shekar, K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10 (Suppl. S5), S629–S641. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Roberts, J.A. Antibiotic dosing during extracorporeal membrane oxygenation: Does the system matter? Curr. Opin. Anaesthesiol. 2020, 33, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M.; et al. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, P.; Öbrink-Hansen, K.; Thorsted, A.; Bue, M.; Tøttrup, M.; Friberg, L.E.; Hardlei, T.F.; Søballe, K.; Gjedsted, J. Population Pharmacokinetics of Meropenem in Plasma and Subcutis from Patients on Extracorporeal Membrane Oxygenation Treatment. Antimicrob. Agents Chemother. 2018, 62, e02390-17. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Ghassabian, S.; Anstey, C.; Wallis, S.C.; Mullany, D.V.; Fung, Y.L.; Fraser, J.F. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: Results from an ex vivo study. Crit. Care 2015, 19, 164. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; Barnett, A.G.; Diab, S.; Wallis, S.C.; Fung, Y.L.; Fraser, J.F. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit. Care 2015, 19, 437. [Google Scholar] [CrossRef]
- Schmidt, M.; Bailey, M.; Kelly, J.; Hodgson, C.; Cooper, D.J.; Scheinkestel, C.; Pellegrino, V.; Bellomo, R.; Pilcher, D. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014, 40, 1256–1266. [Google Scholar] [CrossRef]
- Gélisse, E.; Neuville, M.; de Montmollin, E.; Bouadma, L.; Mourvillier, B.; Timsit, J.F.; Sonneville, R. Extracorporeal membrane oxygenation (ECMO) does not impact on amikacin pharmacokinetics: A case-control study. Intensive Care Med. 2016, 42, 946–948. [Google Scholar] [CrossRef]
- Welsch, C.; Augustin, P.; Allyn, J.; Massias, L.; Montravers, P.; Allou, N. Alveolar and serum concentrations of imipenem in two lung transplant recipients supported with extracorporeal membrane oxygenation. Transpl. Infect. Dis. 2015, 17, 103–105. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Vattanavanit, V.; Wongpoowarak, W.; Nawakitrangsan, M.; Samaeng, M. Pharmacokinetics and Monte Carlo Dosing Simulations of Imipenem in Critically Ill Patients with Life-Threatening Severe Infections During Support with Extracorporeal Membrane Oxygenation. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 735–747. [Google Scholar] [CrossRef]
- Honore, P.M.; Jacobs, R.; Hendrickx, I.; De Waele, E.; Van Gorp, V.; Spapen, H.D. Meropenem therapy in extracorporeal membrane oxygenation patients: An ongoing pharmacokinetic challenge. Crit. Care 2015, 19, 263. [Google Scholar] [CrossRef]
- Cheng, V.; Abdul-Aziz, M.H.; Burrows, F.; Buscher, H.; Cho, Y.J.; Corley, A.; Diehl, A.; Gilder, E.; Jakob, S.M.; Kim, H.S.; et al. Population Pharmacokinetics of Piperacillin and Tazobactam in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation: An ASAP ECMO Study. Antimicrob. Agents Chemother. 2021, 65, e0143821. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Yang, J.H.; Park, H.J.; In, Y.W.; Lee, Y.M.; Cho, Y.H.; Chung, C.R.; Park, C.M.; Jeon, K.; Suh, G.Y. Trough Concentrations of Vancomycin in Patients Undergoing Extracorporeal Membrane Oxygenation. PLoS ONE 2015, 10, e0141016. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Shen, L.J.; Hsu, L.F.; Ko, W.J.; Wu, F.L. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J. Formos. Med. Assoc. 2016, 115, 560–570. [Google Scholar] [CrossRef]
- Marella, P.; Roberts, J.; Hay, K.; Shekar, K. Effectiveness of Vancomycin Dosing Guided by Therapeutic Drug Monitoring in Adult Patients Receiving Extracorporeal Membrane Oxygenation. Antimicrob. Agents Chemother. 2020, 64, e01179-20. [Google Scholar] [CrossRef]
- Donadello, K.; Roberts, J.A.; Cristallini, S.; Beumier, M.; Shekar, K.; Jacobs, F.; Belhaj, A.; Vincent, J.L.; de Backer, D.; Taccone, F.S. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: A matched cohort study. Crit. Care 2014, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Rouse, S.; Elbarbry, F.; Wanek, S.; Grover, V.; Chang, E. Azithromycin Pharmacokinetics in Adults With Acute Respiratory Distress Syndrome Undergoing Treatment With Extracorporeal-Membrane Oxygenation. Ann. Pharmacother. 2016, 50, 72–73. [Google Scholar] [CrossRef]
- Mulla, H.; Peek, G.J.; Harvey, C.; Westrope, C.; Kidy, Z.; Ramaiah, R. Oseltamivir pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation support. Anaesth. Intensive Care 2013, 41, 66–73. [Google Scholar] [CrossRef]
- Eschenauer, G.A.; Lam, S.W. Supratherapeutic oseltamivir levels during continuous dialysis: An expected risk. Intensive Care Med. 2011, 37, 371. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, F.; Luyt, C.E.; Roullet-Renoleau, F.; Nieszkowska, A.; Zahr, N.; Corvol, E.; Fernandez, C.; Antignac, M.; Farinotti, R.; Combes, A. Impact of extracorporeal membrane oxygenation and continuous venovenous hemodiafiltration on the pharmacokinetics of oseltamivir carboxylate in critically ill patients with pandemic (H1N1) influenza. Ther. Drug Monit. 2012, 34, 171–175. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.G.; Corcione, S.; Baietto, L.; Ariaudo, A.; Di Perri, G.; Ranieri, V.M.; D’Avolio, A. Pharmacokinetics of linezolid during extracorporeal membrane oxygenation. Int. J. Antimicrob. Agents 2013, 41, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, M.J.; Conrad, S.A.; Kaufman, D.A.; Rycus, P. Extracorporeal Life Support Organization Task Force on Infections, Extracorporeal Membrane Oxygenation. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr. Crit. Care Med. 2011, 12, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Spriet, I.; Annaert, P.; Meersseman, P.; Hermans, G.; Meersseman, W.; Verbesselt, R.; Willems, L. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J. Antimicrob. Chemother. 2009, 63, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Ihle, F.; Derendorf, H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 2006, 45, 649–663. [Google Scholar] [CrossRef]
- Strunk, A.K.; Ciesek, S.; Schmidt, J.J.; Kühn, C.; Hoeper, M.M.; Welte, T.; Kielstein, J.T. Single- and multiple-dose pharmacokinetics of ethambutol and rifampicin in a tuberculosis patient with acute respiratory distress syndrome undergoing extended daily dialysis and ECMO treatment. Int. J. Infect. Dis. 2016, 42, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, E.S.; Cho, Y.J. Insufficient serum levels of antituberculosis agents during venovenous extracorporeal membrane oxygenation therapy for acute respiratory distress syndrome in a patient with miliary tuberculosis. ASAIO J. 2014, 60, 484–486. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- deBacker, J.; Tamberg, E.; Munshi, L.; Burry, L.; Fan, E.; Mehta, S. Sedation Practice in Extracorporeal Membrane Oxygenation-Treated Patients with Acute Respiratory Distress Syndrome: A Retrospective Study. ASAIO J. 2018, 64, 544–551. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; Ghassabian, S.; Mullany, D.V.; Ziegenfuss, M.; Smith, M.T.; Fung, Y.L.; Fraser, J.F. Sedation during extracorporeal membrane oxygenation-why more is less. Anaesth. Intensive Care 2012, 40, 1067–1069. [Google Scholar] [PubMed]
- Shekar, K.; Roberts, J.A.; Mullany, D.V.; Corley, A.; Fisquet, S.; Bull, T.N.; Barnett, A.G.; Fraser, J.F. Increased sedation requirements in patients receiving extracorporeal membrane oxygenation for respiratory and cardiorespiratory failure. Anaesth. Intensive Care 2012, 40, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Nigoghossian, C.D.; Dzierba, A.L.; Etheridge, J.; Roberts, R.; Muir, J.; Brodie, D.; Schumaker, G.; Bacchetta, M.; Ruthazer, R.; Devlin, J.W. Effect of Extracorporeal Membrane Oxygenation Use on Sedative Requirements in Patients with Severe Acute Respiratory Distress Syndrome. Pharmacotherapy 2016, 36, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Halwick, D.R.; Dodson, B.L.; Thompson, J.E.; Arnold, J.H. Potential drug sequestration during extracorporeal membrane oxygenation: Results from an ex vivo experiment. Intensive Care Med. 2007, 33, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Bhatt-Meht, V.; Annich, G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion 2005, 20, 309–315. [Google Scholar] [CrossRef]
- Bakdach, D.; Akkari, A.; Gazwi, K.; Deloso, F.; Tan, D.; Ibrahim, A.; Abdussalam, A.; Hassan, I. Propofol Safety in Anticoagulated and Nonanticoagulated Patients During Extracorporeal Membrane Oxygenation. ASAIO J. 2021, 67, 201–207. [Google Scholar] [CrossRef]
- Hohlfelder, B.; Szumita, P.M.; Lagambina, S.; Weinhouse, G.; Degrado, J.R. Safety of Propofol for Oxygenator Exchange in Extracorporeal Membrane Oxygenation. ASAIO J. 2017, 63, 179–184. [Google Scholar] [CrossRef]
- Lamm, W.; Nagler, B.; Hermann, A.; Robak, O.; Schellongowski, P.; Buchtele, N.; Bojic, A.; Schmid, M.; Zauner, C.; Heinz, G.; et al. Propofol-based sedation does not negatively influence oxygenator running time compared to midazolam in patients with extracorporeal membrane oxygenation. Int. J. Artif. Organs 2019, 42, 233–240. [Google Scholar] [CrossRef]
- Michaličková, D.; Pokorná, P.; Tibboel, D.; Slanař, O.; Knibbe, C.A.J.; Krekels, E.H.J. Rapid Increase in Clearance of Phenobarbital in Neonates on Extracorporeal Membrane Oxygenation: A Pilot Retrospective Population Pharmacokinetic Analysis. Pediatr. Crit. Care Med. 2020, 21, e707–e715. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- DeGrado, J.R.; Hohlfelder, B.; Ritchie, B.M.; Anger, K.E.; Reardon, D.P.; Weinhouse, G.L. Evaluation of sedatives, analgesics, and neuromuscular blocking agents in adults receiving extracorporeal membrane oxygenation. J. Crit. Care 2017, 37, 1–6. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, H.; Wang, H.; Zhang, H.; An, Y. Comparison between remifentanil and other opioids in adult critically ill patients: A systematic review and meta-analysis. Medicine (Baltimore) 2021, 100, e27275. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Dixon, A.A.; Camporota, L.; Barrett, N.A.; Wan, R.Y.Y. Sedation with alfentanil versus fentanyl in patients receiving extracorporeal membrane oxygenation: Outcomes from a single-centre retrospective study. Perfusion 2020, 35, 104–109. [Google Scholar] [CrossRef]
- Peng, P.W.; Sandler, A.N. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 1999, 90, 576–599. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, B.S.; Dzierba, A.L.; Muir, J.; Der-Nigoghossian, C.; Brodie, D.; Bacchetta, M.; Rietdijk, W.; Bakker, J. Opioid and Benzodiazepine Requirements in Obese Adult Patients Receiving Extracorporeal Membrane Oxygenation. Ann. Pharmacother. 2020, 54, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Landolf, K.M.; Rivosecchi, R.M.; Goméz, H.; Sciortino, C.M.; Murray, H.N.; Padmanabhan, R.R.; Sanchez, P.G.; Harano, T.; Sappington, P.L. Comparison of Hydromorphone versus Fentanyl-based Sedation in Extracorporeal Membrane Oxygenation: A Propensity-Matched Analysis. Pharmacotherapy 2020, 40, 389–397. [Google Scholar] [CrossRef]
- Dean, M. Opioids in renal failure and dialysis patients. J. Pain Symptom Manag. 2004, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Fisquet, S.; Barnett, A.G.; Mullany, D.V.; Ghassabian, S.; Wallis, S.C.; Fung, Y.L.; Smith, M.T.; et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, R194. [Google Scholar] [CrossRef]
- Bovill, J.G.; Sebel, P.S.; Blackburn, C.L.; Oei-Lim, V.; Heykants, J.J. The pharmacokinetics of sufentanil in surgical patients. Anesthesiology 1984, 61, 502–506. [Google Scholar] [CrossRef]
- Scholz, J.; Steinfath, M.; Schulz, M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update. Clin. Pharmacokinet. 1996, 31, 275–292. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; Welch, S.; Buscher, H.; Rudham, S.; Burrows, F.; Ghassabian, S.; Wallis, S.C.; Levkovich, B.; Pellegrino, V.; et al. ASAP ECMO: Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation: A multi-centre study to optimise drug therapy during ECMO. BMC Anesthesiol. 2012, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Yang, S.; Min, K.L.; Kim, D.; Jin, B.H.; Park, C.; Park, M.S.; Wi, J.; Chang, M.J. Population pharmacokinetics of intravenous sufentanil in critically ill patients supported with extracorporeal membrane oxygenation therapy. Crit. Care 2019, 23, 248. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Fellin, R.; Ramzy, D.; Chung, J.S.; Arabia, F.A.; Chan, A.; Ng, D.; D’Attellis, N.; Nurok, M. Role of Methadone in Extracorporeal Membrane Oxygenation: Two Case Reports. J. ExtraCorporeal Technol. 2018, 50, 252–255. [Google Scholar]
- Tellor, B.; Shin, N.; Graetz, T.J.; Avidan, M.S. Ketamine infusion for patients receiving extracorporeal membrane oxygenation support: A case series. F1000Res 2015, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Floroff, C.K.; Hassig, T.B.; Cochran, J.B.; Mazur, J.E. High-Dose Sedation and Analgesia During Extracorporeal Membrane Oxygenation: A Focus on the Adjunctive Use of Ketamine. J. Pain Palliat. Care Pharmacother. 2016, 30, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Dzierba, A.L.; Brodie, D.; Bacchetta, M.; Muir, J.; Wasson, L.; Colabraro, M.; Gannon, W.; Connolly, K.; Biscotti, M.; Rietdijk, W.; et al. Ketamine use in sedation management in patients receiving extracorporeal membrane oxygenation. Intensive Care Med. 2016, 42, 1822–1823. [Google Scholar] [CrossRef]
- Zander, J.; Döbbeler, G.; Nagel, D.; Maier, B.; Scharf, C.; Huseyn-Zada, M.; Jung, J.; Frey, L.; Vogeser, M.; Zoller, M. Piperacillin concentration in relation to therapeutic range in critically ill patients--a prospective observational study. Crit. Care 2016, 20, 79. [Google Scholar] [CrossRef]
- Buck, M.L.; Ksenich, R.A.; Wooldridge, P. Effect of infusing fat emulsion into extracorporeal membrane oxygenation circuits. Pharmacotherapy 1997, 17, 1292–1295. [Google Scholar]
- Buck, M.L.; Wooldridge, P.; Ksenich, R.A. Comparison of methods for intravenous infusion of fat emulsion during extracorporeal membrane oxygenation. Pharmacotherapy 2005, 25, 1536–1540. [Google Scholar] [CrossRef]
- Fuehner, T.; Kuehn, C.; Hadem, J.; Wiesner, O.; Gottlieb, J.; Tudorache, I.; Olsson, K.M.; Greer, M.; Sommer, W.; Welte, T.; et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am. J. Respir. Crit. Care Med. 2012, 185, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Mulla, H.; Lawson, G.; von Anrep, C.; Burke, M.D.; Upton, D.U.; Firmin, R.K.; Killer, H. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion 2000, 15, 21–26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidi, E.; El Nekidy, W.S.; Atallah, B.; Al Zaman, K.; Ghisulal, P.; El Lababidi, R.; Manla, Y.; Ahmed, I.; Sadik, Z.; Taha, A.; et al. Sustaining Life versus Altering Life-Saving Drugs: Insights to Explain the Paradoxical Effect of Extracorporeal Membrane Oxygenation on Drugs. J. Clin. Med. 2023, 12, 3748. https://doi.org/10.3390/jcm12113748
Abidi E, El Nekidy WS, Atallah B, Al Zaman K, Ghisulal P, El Lababidi R, Manla Y, Ahmed I, Sadik Z, Taha A, et al. Sustaining Life versus Altering Life-Saving Drugs: Insights to Explain the Paradoxical Effect of Extracorporeal Membrane Oxygenation on Drugs. Journal of Clinical Medicine. 2023; 12(11):3748. https://doi.org/10.3390/jcm12113748
Chicago/Turabian StyleAbidi, Emna, Wasim S. El Nekidy, Bassam Atallah, Khaled Al Zaman, Praveen Ghisulal, Rania El Lababidi, Yosef Manla, Ihab Ahmed, Ziad Sadik, Ahmed Taha, and et al. 2023. "Sustaining Life versus Altering Life-Saving Drugs: Insights to Explain the Paradoxical Effect of Extracorporeal Membrane Oxygenation on Drugs" Journal of Clinical Medicine 12, no. 11: 3748. https://doi.org/10.3390/jcm12113748