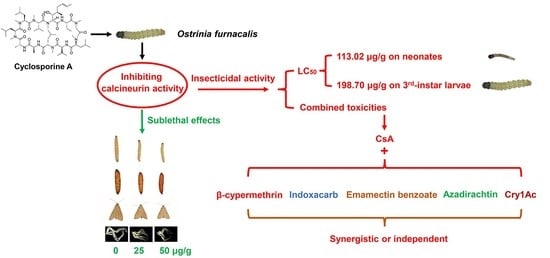
Cyclosporin A as a Potential Insecticide to Control the Asian Corn Borer Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Insects
2.2. CsA and Insecticide Obtention
2.3. Experimental Design
2.4. Insecticidal Activity of CsA against O. furnacalis Larvae
2.5. Effect of Sublethal CsA on Larvae, Pupae, and Adults
2.6. Sublethal Bioassay on Adult Fecundity
2.7. CaN Activity Measurement
2.8. Toxicity of CsA Combined with Other Toxins
2.9. Statistical Analysis
3. Results
3.1. Insecticide Activity of CsA against O. furnacalis Larvae
3.2. Sublethal Effects of CsA on O. furnacalis Larvae
3.3. Post-Exposure Effects of CsA on O. furnacalis Pupae and Adults
3.4. Post-Exposure Effects of CsA on O. furnacalis Reproduction and Eggs
3.5. CsA Inhibits the CaN Activity of O. furnacalis
3.6. Combined Toxicity of CsA and Five Insecticides against Third-Instar Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, F.; Zhang, T.; Bai, S.; Wang, Z.; He, L. Evaluation of Bt corn with pyramided genes on efficacy and insect resistance management for the Asian corn borer in China. PLoS ONE 2016, 11, e0168442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.K.; Le, Q.K.; Tran, T.T.H.; Nguyen, D.V.; Dao, T.H.; Nguyen, T.T.; Truong, X.L.; Nguyen, Q.C.; Pham, H.P.; Phan, T.T.T.; et al. Baseline susceptibility of Asian corn borer (Ostrinia furnacalis (Guenée)) populations in Vietnam to Cry1Ab insecticidal protein. J. Asia Pac. Entomol. 2019, 22, 493–498. [Google Scholar] [CrossRef]
- Rasco, E.T.J.; Mangubat, J.R.; Burgonio, A.B.; Logrono, M.L.; Villegas, V.N.; Fernandez, E.C. Efficacy of insect-protected maize (Bt-11) against Asiatic corn borer Ostrinia furnacalis (Guenee). Philipp. J. Crop Sci. 2005, 33, 82–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ren, Y.; Liu, Y.; Liang, G.; Song, F.; Bai, S.; Wang, J.; Wang, G. Overexpression of a novel Cry1Ie gene confers resistance to Cry1Ac-resistant cotton bollworm in transgenic lines of maize. Plant Cell Tissue Organ Cult. 2013, 115, 151–158. [Google Scholar] [CrossRef]
- Jin, T.; Chang, X.; Gatehouse, A.M.R.; Wang, Z.; Edwards, M.G.; He, K. Downregulation and mutation of a cadherin gene associated with Cry1Ac resistance in the Asian corn borer, Ostrinia furnacalis (Guenée). Toxins 2014, 6, 2676–2693. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Li, D.; Zhang, H.; Mason, C.; Wang, Z.; Lu, X.; Cai, W.; He, K. Seasonal and geographical variation in diapause and cold hardiness of the Asian corn borer, Ostrinia furnacalis. Insect Sci. 2015, 22, 578–586. [Google Scholar] [CrossRef]
- Sparks, T.C.; Wessels, F.J.; Lorsbach, B.A.; Nugent, B.M.; Watson, G.B. The new age of insecticide discovery-the crop protection industry and the impact of natural products. Pestic. Biochem. Phys. 2019, 161, 12–22. [Google Scholar] [CrossRef]
- Murguia-Gonzalez, J.; Presa-Parra, E.; Serna-Lagunes, R.; Andres-Meza, P.; Rosas-Mejia, M.; Garcia-Martinez, M.A. Low concentration of azadirachtin has the same toxic effect as imidacloprid+ lambda-cyhalothrin in workers of two species of leaf-cutter ants. Southwest. Entomol. 2022, 47, 313–324. [Google Scholar] [CrossRef]
- Gao, Q.; Lin, Y.; Wang, X.; Jing, D.; Wang, Z.; He, K.; Bai, S.; Zhang, Y.; Zhang, T. Knockout of ABC transporter ABCG4 gene confers resistance to Cry1 proteins in Ostrinia furnacalis. Toxins 2022, 14, 52. [Google Scholar] [CrossRef]
- Jin, W.; Zhai, Y.; Yang, Y.; Wu, Y.; Wang, X. Cadherin protein is involved in the action of Bacillus thuringiensis Cry1Ac toxin in Ostrinia furnacalis. Toxins 2021, 13, 658. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Huang, J.; Jin, W.; Yang, Y.; Wu, Y. CRISPR-mediated knockout of the ABCC2 gene in Ostrinia furnacalis confers high-level resistance to the Bacillus thuringiensis Cry1Fa toxin. Toxins 2020, 12, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database (APRD). 2019. Available online: http://www.pesticideresistance.org/ (accessed on 16 February 2022).
- Presa-Parra, E.; Llarena-Hernandez, C.; Serna-Lagunes, R.; Briones-Ruiz, G.; Herrera-Solano, A.; Nuñez-Pastrana, R.; Garcia-Martinez, M.A. Effects of concentrations of azadirachtin oil on mortality and post-exposure time of atta mexicana1 leaf-cutter worker ants. Southwest. Entomol. 2021, 46, 83–94. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Elnahas, M.O.; Daba, G.M.; Zohri, A.N.A. Biotechnology and environmental applications of Trichoderma spp. Res. J. Pharmacogn. Phytochem. 2021, 13, 149–157. [Google Scholar] [CrossRef]
- Chighizola, C.B.; Ong, V.H.; Meroni, P.L. The use of cyclosporine A in rheumatology: A 2016 comprehensive review. Clin. Rev. Allergy Immunol. 2016, 52, 401–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Ge, H.; Zhou, S. Cyclosporine A to treat unexplained recurrent spontaneous abortions: A prospective, randomized, double-blind, placebo-controlled, single-center trial. Int. J. Women’s Health 2021, 13, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Ishidoya, S.; Morrissey, J. Role of angiotensin II in the tubulointerstitial fibrosis of obstructive nephropathy. Am. J. Kidney Dis. 1995, 26, 141–146. [Google Scholar] [CrossRef]
- Patel, D.; Wairkar, S. Recent advances in cyclosporine drug delivery: Challenges and opportunities. Drug. Deliv. Transl. Res. 2019, 9, 1067–1081. [Google Scholar] [CrossRef]
- Youn, T.J.; Piao, H.; Kwon, J.S.; Choi, S.Y.; Kim, H.S.; Park, D.G.; Kim, D.W.; Kim, Y.G.; Cho, M.C. Effects of the calcineurin dependent signaling pathway inhibition by cyclosporin A on early and late cardiac remodeling following myocardial infarction. Eur. J. Heart Fail. 2002, 4, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Weiser, J.; Matha, V. The insecticidal activity of cyclosporines on mosquito larvae. J. Invertebr. Pathol. 1988, 51, 92–93. [Google Scholar] [CrossRef]
- Vilcinskas, A.; Kopacek, P.; Jegorov, A.; Vey, A.; Matha, V. Detection of lipophorin as the major cyclosporin-binding protein in the hemolymph of the greater wax moth Galleria mellonella. Comp. Biochem. Physiol. 1997, 117, 41–45. [Google Scholar] [CrossRef]
- Khangembam, R.; Singh, H.; Rath, S.S.; Singh, N.K. Effect of synergists on ivermectin resistance in field populations of Rhipicephalus (Boophilus) microplus from Punjab districts, India. Ticks Tick-Borne Dis. 2018, 9, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.C.; Klafke, G.M.; Júnior, J.R.; Martins, J.R.; da Silva Vaz, I., Jr.; Masuda, A. ABC transporters as a multidrug detoxification mechanism in Rhipicephalus (Boophilus) microplus. Parasitol. Res. 2012, 111, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Boguś, M.I.; Wrońska, A.K.; Kaczmarek, A.; Boguś-Sobocińska, M. In vitro screening of 65 mycotoxins for insecticidal potential. PLoS ONE 2021, 16, e0248772. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Tan, W.; Guo, Y. Improvement of artificial rearing technique of Helicoverpa armigera. Plant Prot. 1999, 25, 15–17. [Google Scholar] [CrossRef]
- Wu, H.; Wan, P.; Huang, S. Toxicity regression calculation method and introduction of corresponding software utilization. J. Anhui Agric. Sci. 2014, 42, 9335–9338, 9340. (In Chinese) [Google Scholar]
- Wei, J.; Guo, Y.; Liang, G.; Wu, K.; Zhang, J.; Tabashnik, B.E.; Li, X. Cross-resistance and interactions between Bt toxins Cry1Ac and Cry2Ab against the cotton bollworm. Sci. Rep. 2015, 5, 7714. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.; Hafeez, M.; Elgizawy, K.; Wang, H.; Zhao, J.; Cai, W.; Ma, W.; Hua, H. Sublethal effects of chlorantraniliprole on Paederus fuscipes (Staphylinidae: Coleoptera), a general predator in paddle field. Environ. Pollut. 2021, 291, 118171. [Google Scholar] [CrossRef]
- Yao, S.; Zhou, S.; Li, X.; Liu, X.; Zhao, W.; Wei, J.; Du, M.; An, S. Transcriptome analysis of Ostrinia furnacalis female pheromone gland: Esters biosynthesis and requirement for mating success. Front. Endocrinol. 2021, 12, 736906. [Google Scholar] [CrossRef]
- Li, Z.; Ni, C.; Xia, C.; Jaw, J.; Wang, Y.; Cao, Y.; Xu, M.; Guo, X. Calcineurin/nuclear factor-κB signaling mediates isoflurane-induced hippocampal neuroinflammation and subsequent cognitive impairment in aged rats. Mol. Med. Rep. 2017, 15, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, L.; Zhang, Y.; Liu, X.; Wei, J.; Xie, Y.; Du, M.; An, S. Calcineurin is required for male sex pheromone biosynthesis and female acceptance. Insect. Mol. Biol. 2018, 27, 373–382. [Google Scholar] [CrossRef]
- Wei, J.; Yang, S.; Zhou, S.; Liu, S.; Cao, P.; Liu, X.; Du, M.; An, S. Suppressing calcineurin activity increases the toxicity of Cry2Ab to Helicoverpa armigera. Pest Manag. Sci. 2021, 77, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yao, X.; Yang, S.; Liu, S.; Zhou, S.; Cen, J.; Liu, X.; Du, M.; Tang, Q.; An, S. Suppression of calcineurin enhances the toxicity of Cry1Ac to Helicoverpa armigera. Front. Microbiol. 2021, 12, 634619. [Google Scholar] [CrossRef] [PubMed]
- Pinos, D.; Wang, Y.; Hernández-Martínez, P.; He, K.; Ferré, J. Alteration of a Cry1A shared binding site in a Cry1Ab-selected colony of Ostrinia furnacalis. Toxins 2022, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Cebolla, J.; Ferreira Dos Santos, R.; Wang, Y.; Caballero, J.; Caballero, P.; He, K.; Jurat-Fuentes, J.L.; Ferré, J. Domain shuffling between Vip3Aa and Vip3Ca: Chimera stability and insecticidal activity against European, American, African, and Asian Pests. Toxins 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.Z.; Quan, Y.; Wang, Z.; Bravo, A.; Soberón, M.; He, K. Characterization of the Cry1Ah resistance in Asian corn borer and its cross-resistance to other Bacillus thuringiensis toxins. Sci. Rep. 2018, 8, 234. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Y.; Wang, Z.; Bravo, A.; Soberón, M.; He, K. Genetic basis of Cry1F resistance in a laboratory selected Asian corn borer strain and its cross-resistance to other Bacillus thuringiensis toxins. PLoS ONE 2016, 11, e0161189. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Z.; Zhang, J.; He, K.; Ferry, N.; Gatehouse, A.M.R. Cross-resistance of Cry1Ab-selected Asian corn borer to other Cry toxins. J. Appl. Entomol. 2010, 134, 429–438. [Google Scholar] [CrossRef]
- Zhang, T.; He, M.; Gatehouse, A.M.; Wang, Z.; Edwards, M.G.; Li, Q.; He, K. Inheritance patterns, dominance and cross-resistance of Cry1Ab- and Cry1Ac-selected Ostrinia furnacalis (Guenée). Toxins 2014, 6, 2694–2707. [Google Scholar] [CrossRef] [Green Version]
- Mostafiz, M.M.; Hassan, E.; Shim, J.K.; Lee, K.Y. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, L.; Yang, S.; Xie, B.; An, S.; Liang, G. Assessment of the lethal and sublethal effects by spinetoram on cotton bollworm. PLoS ONE 2018, 9, e0204154. [Google Scholar] [CrossRef]
- Survase, S.A.; Kagliwal, L.D.; Annapure, U.S.; Singhal, R.S. Cyclosporin A—A review on fermentative production, downstream processing and pharmacological applications. Biotechnol. Adv. 2011, 29, 418–435. [Google Scholar] [CrossRef]
- Sasamura, S.; Kobayashi, M.; Muramatsu, H.; Yoshimura, S.; Kinoshita, T.; Ohki, H.; Okada, K.; Deai, Y.; Yamagishi, Y.; Hashimoto, M. Bioconversion of FR901459, a novel derivative of cyclosporin A, by Lentzea sp. 7887. J. Antibiot. 2015, 68, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T. Neurophysiological effects of insecticides. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Robert, K., Ed.; Academic Press: New York, NY, USA, 2010; Volume 1, pp. 799–817. [Google Scholar]
- Wing, K.D.; Sacher, M.; Kagaya, Y.; Tsurubuchi, Y.; Mulderig, L.; Connair, M.; Schnee, M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Prot. 2000, 19, 537–545. [Google Scholar] [CrossRef]
- Yu, S.J. The Mode of Action of Insecticides. In The Toxicology and Biochemistry of Insecticides, 2nd ed.; Yu, S.J., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 133–167. [Google Scholar]
- Ishaaya, I.; Kontsedalov, S.; Horowitz, A.R. Emamectin, a novel insecticide for controlling field crop pests. Pest Manag. Sci. 2002, 58, 1091–1095. [Google Scholar] [CrossRef]
- Park, J.M. Rapid development of life-threatening emamectin benzoate poisoning. Emerg. Med. 2018, 50, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Bauer, L.S.; Pankratz, H.S. Ultrastructural effects of Bacillus thuringiensis var. San Diego on midgut cells of the cottonwood leaf beetle. J. Invertebr. Pathol. 1992, 60, 15–25. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Mordue Luntz, A.J. The effect of azadirachtin on the midgut histology of the locusts, Schistocerca gregaria and Locusta migratoria. Tissue Cell 1993, 25, 875–884. [Google Scholar] [CrossRef]
- Nathan, S.S.; Kalaivani, K.; Chung, P.G. The effects of azadirachtin and nucleopolyhedrovirus on midgut enzymatic profile of Spodoptera litura Fab. (Lepidoptera: Noctuidae). Pestic. Biochem. Phys. 2005, 83, 46–57. [Google Scholar] [CrossRef]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri-Kordestani, L.; Basseville, A.; Kurdziel, K.; Fojo, A.T.; Bates, S.E. Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat. 2012, 15, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Campos, B.; Altenburger, R.; Gómez, C.; Lacorte, S.; Piña, B.; Barata, C.; Luckenbach, T. First evidence for toxic defense based on the multixenobiotic resistance (MXR) mechanism in Daphnia magna. Aquat. Toxicol. 2014, 148, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-binding cassette (ABC) transporters: Roles in xenobiotic detoxification and Bt insecticidal activity. Int. J. Mol. Sci. 2019, 20, 2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, M.W.; Galat, A.; Uehling, D.E.; Schreiber, S.L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature 1989, 341, 758–760. [Google Scholar] [CrossRef]
- Harding, M.W.; Handschumacher, R.E. Cyclophilin, a primary molecular target for cyclosporine. Structural and functional implications. Transplantation 1988, 46, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, L.; Yao, S.; Yang, S.; Zhou, S.; Liu, X.; Du, M.; An, S. Calcineurin-modulated antimicrobial peptide expression is required for the development of Helicoverpa armigera. Front. Physiol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiolka, M.J. Immunosuppressive effect of cyclosporin A on insect humoral immune response. J. Invertebr. Pathol. 2008, 98, 287–292. [Google Scholar] [CrossRef]
- Du, M.; Liu, X.; Ma, N.; Liu, X.; Wei, J.; Yin, X.; Zhou, S.; Rafaeli, A.; Song, Q.; An, S. Calcineurin-mediated dephosphorylation of acetyl-coA carboxylase is required for pheromone biosynthesis activating neuropeptide (PBAN)-induced sex pheromone biosynthesis in Helicoverpa armigera. Mol. Cell Proteom. 2017, 16, 2138–2152. [Google Scholar] [CrossRef]




| Larvae | LC50 (95% CLa) (μg/g) | LC95 (95% CL) (μg/g) | Slope ± SE | χ2 | pb |
|---|---|---|---|---|---|
| Neonates | 113.02 (60.38–289.71) | 1,107.64 (381.54–38,337.57) | 1.66 ± 0.15 | 20.44 | <0.01 |
| Third-instar | 198.70 (134.36–317.63) | 2457.28 (1096.90–12,480.19) | 1.51 ± 0.14 | 7.38 | 0.117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Li, S.; Wang, K.; Yin, X.; Wang, Y.; Du, M.; Wei, J.; An, S. Cyclosporin A as a Potential Insecticide to Control the Asian Corn Borer Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae). Insects 2022, 13, 965. https://doi.org/10.3390/insects13100965
Sun C, Li S, Wang K, Yin X, Wang Y, Du M, Wei J, An S. Cyclosporin A as a Potential Insecticide to Control the Asian Corn Borer Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae). Insects. 2022; 13(10):965. https://doi.org/10.3390/insects13100965
Chicago/Turabian StyleSun, Chengxian, Shunjia Li, Kai Wang, Xinming Yin, Yanmei Wang, Mengfang Du, Jizhen Wei, and Shiheng An. 2022. "Cyclosporin A as a Potential Insecticide to Control the Asian Corn Borer Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae)" Insects 13, no. 10: 965. https://doi.org/10.3390/insects13100965