Imidacloprid Movement into Fungal Conidia Is Lethal to Mycophagous Beetles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
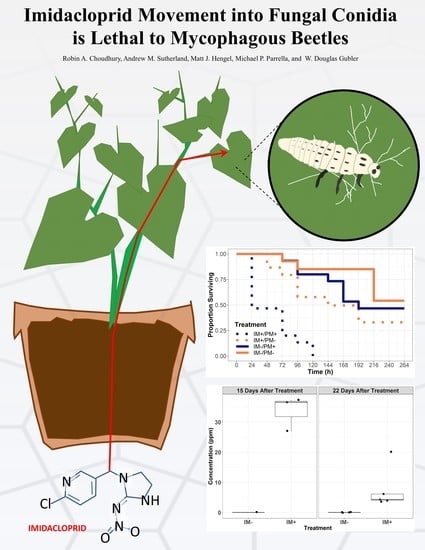
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Xiao, D.; Li, J.; Chen, Z.; Biondi, A.; Desneux, N.; Gao, X.; Song, D. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jang, M.J.; Lee, H.A.; Lee, J.H. Toxicity of pesticides to mycophagous ladybird, Illeis koebelei Timberlake (Coleoptera: Coccinellidae: Halyziini). Korean J. Pestic. Sci. 2017, 21, 364–372. [Google Scholar] [CrossRef]
- Skouras, P.J.; Brokaki, M.; Stathas, G.J.; Demopoulos, V.; Louloudakis, G.; Margaritopoulos, J.T. Lethal and sub-lethal effects of imidacloprid on the aphidophagous coccinellid hippodamia variegata. Chemosphere 2019, 229, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tappert, L.; Pokorny, T.; Hofferberth, J.; Ruther, J. Sublethal doses of imidacloprid disrupt sexual communication and host finding in a parasitoid wasp. Sci. Rep. 2017, 7, 42756. [Google Scholar] [CrossRef] [Green Version]
- Put, K.; Bollens, T.; Wäckers, F.; Pekas, A. Non-target effects of commonly used plant protection products in roses on the predatory mite Euseius gallicus Kreiter & Tixier (Acari: Phytoseidae). Pest Manag. Sci. 2016, 72, 1373–1380. [Google Scholar]
- Bonmatin, J.; Moineau, I.; Charvet, R.; Fleche, C.; Colin, M.; Bengsch, E. A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens. Anal. Chem. 2003, 75, 2027–2033. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Hoffmann, E.J.; Castle, S.J. Imidacloprid in melon guttation fluid: A potential mode of exposure for pest and beneficial organisms. J. Econ. Entomol. 2012, 105, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Grafton-Cardwell, E.E.; Gu, P. Conserving vedalia beetle, Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae), in citrus: A continuing challenge as new insecticides gain registration. J. Econ. Entomol. 2003, 96, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in excretion product of phloem-feeding insects kill beneficial insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, A.M.; Parrella, M.P. Mycophagy in Coccinellidae: Review and synthesis. Biol. Control 2009, 51, 284–293. [Google Scholar] [CrossRef]
- Davidson, W. Observations on Psyllobora taedata LeConte, a coccinellid attacking mildews. Entomol. News 1921, 32, 83–89. [Google Scholar]
- Therneau, T.M. A Package for Survival Analysis in S, R Package Version 2.38. 2015. Available online: https://cran.r-project.org/web/packages/survival/ (accessed on 10 January 2020).
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Stoner, K.A.; Eitzer, B.D. Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS ONE 2012, 7, e39114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krischik, V.; Rogers, M.; Gupta, G.; Varshney, A. Soil-applied imidacloprid translocates to ornamental flowers and reduces survival of adult Coleomegilla maculata, Harmonia axyridis, and Hippodamia convergens lady beetles, and larval Danaus plexippus and Vanessa cardui butterflies. PLoS ONE 2015, 10, e0119133. [Google Scholar] [CrossRef]
- Mizell, R.F.; Sconyers, M.C. Toxicity of imidacloprid to selected arthropod predators in the laboratory. Fla. Entomol. 1992, 75, 277–280. [Google Scholar] [CrossRef]
- Skouras, P.J.; Stathas, G.J.; Voudouris, C.C.; Darras, A.I.; Tsitsipis, J.A.; Margaritopoulos, J.T. Effect of synthetic insecticides on the larvae of Coccinella septempunctata from Greek populations. Phytoparasitica 2017, 45, 165–173. [Google Scholar] [CrossRef]
- Sutherland, A.M.; Parrella, M.P. Quantification of powdery mildew removal by the mycophagous beetle Psyllobora vigintimaculata (Coleoptera: Coccinellidae). IOBC WPRS Bull. 2006, 29, 281. [Google Scholar]
- Peduto, F.; Sutherland, A.; Hand, E.; Broome, J.; Parikh, P.; Bettiga, L.; Smith, R.; Mahaffee, W.; Gubler, W. Comparing the efficiency of visual scouting, spore trapping systems and a bioindicator for early detection of Erysiphe necator in California vineyards. Phytopathology 2011, 101, S139. [Google Scholar]
- Parrella, M.P.; Lewis, E. Biological control in greenhouse and nursery production: Present status and future directions. Am. Entomol. 2017, 63, 237–250. [Google Scholar] [CrossRef]
- Sutherland, A.M.; Gubler, W.D.; Parrella, M.P. Effects of fungicides on a mycophagous coccinellid may represent integration failure in disease management. Biol. Control 2010, 54, 292–299. [Google Scholar] [CrossRef]
- Xiao, D.; Zhao, J.; Guo, X.; Chen, H.; Qu, M.; Zhai, W.; Desneux, N.; Biondi, A.; Zhang, F.; Wang, S. Sublethal effects of imidacloprid on the predatory seven-spot ladybird beetle Coccinella septempunctata. Ecotoxicology 2016, 25, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Wyckhuys, K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, R.A.; Sutherland, A.M.; Hengel, M.J.; Parrella, M.P.; Gubler, W.D. Imidacloprid Movement into Fungal Conidia Is Lethal to Mycophagous Beetles. Insects 2020, 11, 496. https://doi.org/10.3390/insects11080496
Choudhury RA, Sutherland AM, Hengel MJ, Parrella MP, Gubler WD. Imidacloprid Movement into Fungal Conidia Is Lethal to Mycophagous Beetles. Insects. 2020; 11(8):496. https://doi.org/10.3390/insects11080496
Chicago/Turabian StyleChoudhury, Robin A., Andrew M. Sutherland, Matt J. Hengel, Michael P. Parrella, and W. Douglas Gubler. 2020. "Imidacloprid Movement into Fungal Conidia Is Lethal to Mycophagous Beetles" Insects 11, no. 8: 496. https://doi.org/10.3390/insects11080496