Chemical Stability of Metal Halide Perovskite Detectors
Abstract
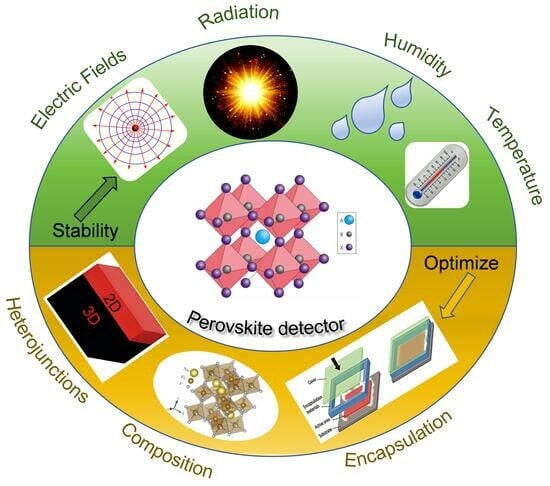
:1. Introduction
2. Degradation Factors Impacting MHP Detectors
2.1. Radiation
2.1.1. Ultraviolet and Visible Light

2.1.2. X-ray
2.1.3. γ-ray
2.1.4. Electron Beams
2.1.5. Proton Beams

2.1.6. Neutron Beams
2.2. Humidity
2.3. Temperature
2.4. Electric Fields
3. Stability Optimization of MHP Detectors
3.1. Composition

3.2. Heterojunctions
3.3. Encapsulation

4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Corti, M.; Bonomi, S.; Chiara, R.; Romani, L.; Quadrelli, P.; Malavasi, L. Application of metal halide perovskites as photocatalysts in organic reactions. Inorganics 2021, 9, 56. [Google Scholar] [CrossRef]
- Xiao, S.; Qian, W.; Yang, S. Interfaced structures between halide perovskites: From basics to construction to optoelectronic applications. Adv. Energy Mater. 2023, 13, 2201472. [Google Scholar] [CrossRef]
- Xu, F.; Zou, Y.; Dai, Y.; Li, M.; Li, Z. Halide perovskites and high-pressure technologies: A fruitful encounter. Mater. Chem. Front. 2023, 7, 2102–2119. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, H.; Wang, H.; Song, Y.; Li, W.; Zhu, L.; Xie, X.; Xiao, S.; Chen, H. Carbon-based Sb2 (S, Se)3 solar cells. Inorganics 2023, 11, 159. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, P.; Xu, T.; Zhou, H.; Bai, Y.; Chen, Q. Chemical approaches for electronic doping in photovoltaic materials beyond crystalline silicon. Chem. Soc. Rev. 2022, 51, 10016–10063. [Google Scholar] [CrossRef]
- Bu, H.; He, C.; Xu, Y.; Xing, L.; Liu, X.; Ren, S.; Yi, S.; Chen, L.; Wu, H.; Zhang, G.; et al. Emerging new-generation detecting and sensing of metal halide perovskites. Adv. Electron. Mater. 2022, 8, 2101204. [Google Scholar] [CrossRef]
- He, Y.; Hadar, I.; Kanatzidis, M.G. Detecting ionizing radiation using halide perovskite semiconductors processed through solution and alternative methods. Nat. Photonics 2022, 16, 14–26. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Rajagopal, A.; Yang, Z.; Jo, S.B.; Braly, I.L.; Liang, P.W.; Hillhouse, H.W.; Jen, A.K.Y. Highly efficient perovskite–perovskite tandem solar cells reaching 80% of the theoretical limit in photovoltage. Adv. Mater. 2017, 29, 1702140. [Google Scholar] [CrossRef]
- Jacak, J.E.; Jacak, W.A. Routes for metallization of perovskite solar cells. Materials 2022, 15, 2254. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, N.; Chen, S.; Guo, F.; Xu, B. Recent progress on synthesis, intrinsic properties and optoelectronic applications of perovskite single crystals. Adv. Funct. Mater. 2023, 33, 2214339. [Google Scholar] [CrossRef]
- Bao, C.; Xu, W.; Yang, J.; Bai, S.; Teng, P.; Yang, Y.; Wang, J.; Zhao, N.; Zhang, W.; Huang, W.; et al. Bidirectional optical signal transmission between two identical devices using perovskite diodes. Nat. Electron. 2020, 3, 156–164. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, Y.; Ouyang, D.; Zhang, N.; Wang, J.; Murthy, A.A.; Spanopoulos, I.; Islam, S.M.; Tu, Q.; Xing, G.; et al. Recent development of halide perovskite materials and devices for ionizing radiation detection. Chem. Rev. 2023, 123, 1207–1261. [Google Scholar] [CrossRef] [PubMed]
- Dudipala, K.R.; Le, T.-H.; Nie, W.; Hoye, R.L.Z. Halide perovskites and their derivatives for efficient, high-resolution direct radiation detection: Design strategies and applications. Adv. Mater. 2023, 35, 2304523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, Z.; Lan, W.; Wang, Q.; Ding, L.; Jin, Z. Halide perovskite, a potential scintillator for x-ray detection. Small Methods 2020, 4, 2000506. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Chai, Z.; Wang, Y.; Wang, S. Perovskite scintillators for improved X-ray detection and imaging. Angew. Chem. Int. Edit. 2023, 62, e202304638. [Google Scholar] [CrossRef]
- He, Y.; Lin, Z.; Wang, J.; Zhang, K.; Xu, X.; Li, Y.; Huang, X.; Ma, T.; Xiao, S.; Yang, S. A heat-liquefiable solid precursor for ambient growth of perovskites with high tunability, performance and stability. Small Methods 2022, 6, 2200384. [Google Scholar] [CrossRef]
- Hu, H.; Niu, G.; Zheng, Z.; Xu, L.; Liu, L.; Tang, J. Perovskite semiconductors for ionizing radiation detection. EcoMat 2022, 4, e12258. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding degradation mechanisms and improving stability of perovskite photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; van Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From defects to degradation: A mechanistic understanding of degradation in perovskite solar cell devices and modules. Adv. Energy Mater. 2020, 10, 1904054. [Google Scholar] [CrossRef]
- Meng, X.; Tian, X.; Zhang, S.; Zhou, J.; Zhang, Y.; Liu, Z.; Chen, W. In situ characterization for understanding the degradation in perovskite solar cells. Sol. RRL 2022, 6, 2200772. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The role of oxygen in the degradation of methylammonium lead trihalide perovskite photoactive layers. Angew. Chem. Int. Ed. 2015, 54, 8208–8212. [Google Scholar] [CrossRef]
- Nickel, N.H.; Lang, F.; Brus, V.V.; Shargaieva, O.; Rappich, J. Unraveling the light-induced degradation mechanisms of CH3NH3PbI3 perovskite films. Adv. Electron. Mater. 2017, 3, 1700158. [Google Scholar] [CrossRef]
- Gottesman, R.; Gouda, L.; Kalanoor, B.S.; Haltzi, E.; Tirosh, S.; Rosh-Hodesh, E.; Tischler, Y.; Zaban, A.; Quarti, C.; Mosconi, E.; et al. Photoinduced reversible structural transformations in free-standing CH3NH3PbI3 perovskite films. J. Phys. Chem. Lett. 2015, 6, 2332–2338. [Google Scholar] [CrossRef]
- Nie, W.; Blancon, J.-C.; Neukirch, A.J.; Appavoo, K.; Tsai, H.; Chhowalla, M.; Alam, M.A.; Sfeir, M.Y.; Katan, C.; Even, J.; et al. Light-activated photocurrent degradation and self-healing in perovskite solar cells. Nat. Commun. 2016, 7, 11574. [Google Scholar] [CrossRef]
- Domanski, K.; Roose, B.; Matsui, T.; Saliba, M.; Turren-Cruz, S.-H.; Correa-Baena, J.-P.; Roldan-Carmona, C.; Richardson, G.; Foster, J.M.; De Angelis, F.; et al. Migration of cations induces reversible performance losses over day/night cycling in perovskite solar cells. Energy Environ. Sci. 2017, 10, 604–613. [Google Scholar] [CrossRef]
- Yang, X.; Yan, X.; Wang, W.; Zhu, X.; Li, H.; Ma, W.; Sheng, C. Light induced metastable modification of optical properties in CH3NH3PbI3− xBrx perovskite films: Two-step mechanism. Org. Electron. 2016, 34, 79–83. [Google Scholar] [CrossRef]
- Kirschner, M.S.; Diroll, B.T.; Guo, P.; Haryey, S.M.; Helweh, W.; Flanders, N.C.; Brumberg, A.; Watkins, N.E.; Leonard, A.A.; Evans, A.M.; et al. Photoinduced, reversible phase transitions in all-inorganic perovskite nanocrystals. Nat. Commun. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; You, L.; Wang, S.; Ku, Z.; Fan, H.; Schmidt, D.; Rusydi, A.; Chang, L.; Wang, L.; Ren, P.; et al. Giant photostriction in organic-inorganic lead halide perovskites. Nat. Commun. 2016, 7, 11193. [Google Scholar] [CrossRef] [PubMed]
- Conings, B.; Baeten, L.; De Dobbelaere, C.; D’Haen, J.; Manca, J.; Boyen, H.-G. Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach. Adv. Mater. 2014, 26, 2041–2046. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Marin, T.W. Charge trapping in photovoltaically active perovskites and related halogenoplumbate compounds. J. Phys. Chem. Lett. 2014, 5, 1066–1071. [Google Scholar] [CrossRef]
- Philippe, B.; Park, B.-W.; Lindblad, R.; Oscarsson, J.; Ahmadi, S.; Johansson, E.M.J.; Rensmo, H. Chemical and electronic structure characterization of lead halide perovskites and stability behavior under different exposures—A photoelectron spectroscopy investigation. Chem. Mater. 2015, 27, 1720–1731. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lo, W.-C.; Li, J.-X.; Wang, Y.-K.; Tang, J.-F.; Fong, Z.-Y. In situ XPS investigation of the X-ray-triggered decomposition of perovskites in ultrahigh vacuum condition. NPJ Mat. Degrad. 2021, 5, 13. [Google Scholar] [CrossRef]
- Svanstrom, S.; Garcia Fernandez, A.; Sloboda, T.; Jacobsson, T.J.; Rensmo, H.; Cappel, U.B. X-ray stability and degradation mechanism of lead halide perovskites and lead halides. Phys. Chem. Chem. Phys. 2021, 23, 12479–12489. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fei, C.; Kandlakunta, P.; Zhao, L.; Ni, Z.; Cao, L.R.; Huang, J. Origin of the X-ray-induced damage in perovskite solar cells. IEEE Trans. Nucl. Sci. 2022, 69, 1850–1856. [Google Scholar] [CrossRef]
- Motoki, K.; Miyazawa, Y.; Kobayashi, D.; Ikegami, M.; Miyasaka, T.; Yamamoto, T.; Hirose, K. Degradation of CH3NH3PbI3 perovskite due to soft X-ray irradiation as analyzed by an X-ray photoelectron spectroscopy time-dependent measurement method. J. Appl. Phys. 2017, 121, 085501. [Google Scholar] [CrossRef]
- Armaroli, G.; Ferlauto, L.; Ledee, F.; Lini, M.; Ciavatti, A.; Kovtun, A.; Borgatti, F.; Calabrese, G.; Milita, S.; Fraboni, B.; et al. X-ray-induced modification of the photophysical properties of MAPbBr3 single crystals. ACS Appl. Mater. Interfaces 2021, 13, 58301–58308. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ecker, B.R.; Wei, H.; Huang, J.; Gao, Y. Environmental surface stability of the MAPbBr3 single crystal. J. Phys. Chem. C 2018, 122, 3513–3522. [Google Scholar] [CrossRef]
- Garcia-Fernandez, A.; Kammlander, B.; Riva, S.; Rensmo, H.; Cappel, U.B. Composition dependence of X-ray stability and degradation mechanisms at lead halide perovskite single crystal surfaces. Phys. Chem. Chem. Phys. 2024, 26, 1000–1010. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Z.; Xue, S.; Kandlakunta, P.; Cao, L.; Huang, J. Organohalide lead perovskites: More stable than glass undergamma-ray radiation. Adv. Mater. 2019, 31, 0935–9648. [Google Scholar] [CrossRef]
- Boldyreva, A.G.; Akbulatov, A.F.; Tsarev, S.A.; Luchkin, S.Y.; Zhidkov, I.S.; Kurmaev, E.Z.; Stevenson, K.J.; Petrov, V.G.; Troshin, P.A. Γ-ray-induced degradation in the triple-cation perovskite solar cells. J. Phys. Chem. Lett. 2019, 10, 813–818. [Google Scholar] [CrossRef]
- Yang, K.; Huang, K.; Li, X.; Zheng, S.; Hou, P.; Wang, J.; Guo, H.; Song, H.; Li, B.; Li, H.; et al. Radiation tolerance of perovskite solar cells under gamma ray. Org. Electron. 2019, 71, 79–84. [Google Scholar] [CrossRef]
- Boldyreva, A.G.; Frolova, L.A.; Zhidkov, I.S.; Gutsev, L.G.; Kurmaev, E.Z.; Ramachandran, B.R.; Petrov, V.G.; Stevenson, K.J.; Aldoshin, S.M.; Troshin, P.A. Unravelling the material composition effects on the gamma ray stability of lead halide perovskite solar cells: MAPbI3 breaks the records. J. Phys. Chem. Lett. 2020, 11, 2630–2636. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, H.; Nie, J.; Shao, W.; Wang, X.; Zhang, B.; Ouyang, X. Effect of methylammonium lead tribromide perovskite based-photoconductor under gamma photons radiation. Radiat. Phys. Chem. 2021, 181, 109337. [Google Scholar] [CrossRef]
- Gao, L.; Tao, K.; Sun, J.-L.; Yan, Q. Gamma-ray radiation stability of mixed-cation lead mixed-halide perovskite single crystals. Adv. Opt. Mater. 2022, 10, 2102069. [Google Scholar] [CrossRef]
- Gao, L.; Sun, J.-L.; Li, Q.; Yan, Q. γ-ray radiation hardness of CsPbBr3 single crystals and single-carrier devices. ACS Appl. Mater. Interfaces 2022, 14, 37904–37915. [Google Scholar] [CrossRef]
- De Siena, M.C.; Klepov, V.V.; Stepanoff, S.P.; Bayikadi, K.S.; Pan, L.; Pandey, I.R.; Karki, S.; Chung, D.Y.; Wolfe, D.E.; Kanatzidis, M.G. Extreme γ-ray radiation tolerance of spectrometer-grade CsPbBr3 perovskite detectors. Adv. Mater. 2023, 35, 2303244. [Google Scholar] [CrossRef]
- Zaffalon, M.L.; Cova, F.; Liu, M.; Cemmi, A.; Di Sarcina, I.; Rossi, F.; Carulli, F.; Erroi, A.; Roda, C.; Perego, J.; et al. Extreme γ-ray radiation hardness and high scintillation yield in perovskite nanocrystals. Nat. Photonics 2022, 16, 860–868. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Sun, J.-L.; Yan, Q. Gamma-ray irradiation stability of zero-dimensional cs3cu2i5 metal halide scintillator single crystals. J. Phys. Chem. Lett. 2023, 14, 1165–1173. [Google Scholar] [CrossRef]
- Ozerova, V.V.; Emelianov, N.A.; Kiryukhin, D.P.; Kushch, P.P.; Shilov, G.V.; Kichigina, G.A.; Aldoshin, S.M.; Frolova, L.A.; Troshin, P.A. Exploring the limits: Degradation behavior of lead halide perovskite films under exposure to ultrahigh doses of γ rays of up to 10 MGy. J. Phys. Chem. Lett. 2023, 14, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Shamsi, J.; Palazon, F.; Imran, M.; Akkerman, Q.A.; Park, S.; Bertoni, G.; Prato, M.; Brescia, R.; Manna, L. In situ transmission electron microscopy study of electron beam-induced transformations in colloidal cesium lead halide perovskite nanocrystals. ACS Nano 2017, 11, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z. Investigating chemical and structural instabilities of lead halide perovskite induced by electron beam irradiation. Micron 2019, 116, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Syafutra, H.; Yun, J.-H.; Yoshie, Y.; Lyu, M.; Takeda, S.N.; Nakamura, M.; Wang, L.; Jung, M.-C. Surface degradation mechanism on CH3NH3PbBr3 hybrid perovskite single crystal by a grazing e-beam irradiation. Nanomaterials 2020, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-S.; Kelzenberg, M.D.; Espinet-Gonzalez, P.; Mann, C.; Walker, D.; Naqavi, A.; Vaidya, N.; Warmann, E.; Atwater, H.A. Effects of electron and proton radiation on perovskite solar cells for space solar power application. In Proceedings of the IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017; pp. 1248–1252. [Google Scholar]
- Miyazawa, Y.; Kim, G.M.; Ishii, A.; Ikegami, M.; Miyasaka, T.; Suzuki, Y.; Yamamoto, T.; Ohshima, T.; Kanaya, S.; Toyota, H.; et al. Evaluation of damage coefficient for minority-carrier diffusion length of triple-cation perovskite solar cells under 1 mev electron irradiation for space applications. J. Phys. Chem. C 2021, 125, 13131–13137. [Google Scholar] [CrossRef]
- Lang, F.; Nickel, N.H.; Bundesmann, J.; Seidel, S.; Denker, A.; Albrecht, S.; Brus, V.V.; Rappich, J.; Rech, B.; Landi, G.; et al. Radiation hardness and self-healing of perovskite solar cells. Adv. Mater. 2016, 28, 8726–8731. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Ikegami, M.; Chen, H.-W.; Ohshima, T.; Imaizumi, M.; Hirose, K.; Miyasaka, T. Tolerance of perovskite solar cell to high-energy particle irradiations in space environment. iScience 2018, 2, 148–155. [Google Scholar] [CrossRef]
- Afshari, H.; Chacon, S.A.; Durant, B.K.; Crawford, R.; Rout, B.; Eperon, G.E.; Sellers, I.R. Radiation tolerance, high temperature stability, and self-healing of triple halide perovskite solar cells. In Proceedings of the IEEE 49th Photovoltaics Specialists Conference (PVSC), Philadelphia, PA, USA, 5–10 June 2022; p. 836. [Google Scholar]
- Huang, H.; Guo, L.; Zhao, Y.; Peng, S.; Ma, W.; Wang, X.; Xue, J. Radiation-tolerant proton detector based on the mapbbr3 single crystal. ACS Appl. Electron. Mater. 2023, 5, 381–387. [Google Scholar] [CrossRef]
- Paterno, G.M.; Robbiano, V.; Santarelli, L.; Zampetti, A.; Cazzaniga, C.; Sakai, V.G.; Cacialli, F. Perovskite solar cell resilience to fast neutrons. Sustain. Energy Fuels 2019, 3, 2561–2566. [Google Scholar] [CrossRef]
- De Rossi, F.; Taheri, B.; Bonomo, M.; Gupta, V.; Renno, G.; Nia, N.Y.; Rech, P.; Frost, C.; Cazzaniga, C.; Quagliotto, P.; et al. Neutron irradiated perovskite films and solar cells on pet substrates. Nano Energy 2022, 93, 106879. [Google Scholar] [CrossRef]
- Rafiei Rad, R.; Azizollah Ganji, B.; Taghavinia, N. 4-tert-butyl pyridine additive for moisture-resistant wide bandgap perovskite solar cells. Opt. Mater. 2022, 123, 111876. [Google Scholar] [CrossRef]
- Strachala, D.; Hylsky, J.; Vanek, J. Influence of moisture on perovskite photovoltaic cells. ECS Trans. 2017, 81, 191–198. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, S.; Tress, W. A review on the stability of inorganic metal halide perovskites: Challenges and opportunities for stable solar cells. Energy Environ. Sci. 2021, 14, 2090–2113. [Google Scholar] [CrossRef]
- Sun, K.; Mueller-Buschbaum, P. Shedding light on the moisture stability of halide perovskite thin films. Energy Technol. 2023, 11, 2201475. [Google Scholar] [CrossRef]
- Hsu, R.-Y.; Liang, Y.-J.; Hung, Y.-J.; Lin, Y.-C. Impact of humidity in triple cation perovskite solar cells: Surface analysis. Mat. Sci. Semicon. Proc. 2022, 152, 107100. [Google Scholar] [CrossRef]
- Ho, K.; Wei, M.; Sargent, E.H.; Walker, G.C. Grain transformation and degradation mechanism of formamidinium and cesium lead iodide perovskite under humidity and light. ACS Energy Lett. 2021, 6, 934–940. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Liu, Y.; Deng, Y.; Bai, Y.; Dong, Q.; Huang, J. Scaling behavior of moisture-induced grain degradation in polycrystalline hybrid perovskite thin films. Energy Environ. Sci. 2017, 10, 516–522. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Phys. Chem. A 2014, 2, 705–710. [Google Scholar]
- Fan, Z.; Xiao, H.; Wang, Y.; Zhao, Z.; Lin, Z.; Cheng, H.-C.; Lee, S.-J.; Wang, G.; Feng, Z.; Goddard, W.A., III; et al. Layer-by-layer degradation of methylammonium lead tri-iodide perovskite microplates. Joule 2017, 1, 548–562. [Google Scholar] [CrossRef]
- Howard, J.M.; Tennyson, E.M.; Barik, S.; Szostak, R.; Waks, E.; Toney, M.F.; Nogueira, A.F.; Neves, B.R.A.; Leite, M.S. Humidity-induced photoluminescence hysteresis in variable Cs/Br ratio hybrid perovskites. J. Phys. Chem. Lett. 2018, 9, 3463–3469. [Google Scholar] [CrossRef]
- Zheng, C.; Rubel, O. Unraveling the water degradation mechanism of CH3NH3PbI3. J. Phys. Chem. C 2019, 123, 19385–19394. [Google Scholar] [CrossRef]
- Dastidar, S.; Egger, D.A.; Tan, L.Z.; Cromer, S.B.; Dillon, A.D.; Liu, S.; Kronik, L.; Rappe, A.M.; Fafarman, A.T. High chloride doping levels stabilize the perovskite phase of cesium lead iodide. Nano Lett. 2016, 16, 3563–3570. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium lead halide perovskites with improved stability for tandem solar cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Abdelmageed, G.; Mackeen, C.; Hellier, K.; Jewell, L.; Seymour, L.; Tingwald, M.; Bridges, F.; Zhang, J.Z.; Carter, S. Effect of temperature on light induced degradation in methylammonium lead iodide perovskite thin films and solar cells. Sol. Energy Mater. Sol. Cells 2018, 174, 566–571. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Martynenko, V.M.; Frolova, L.A.; Dremova, N.N.; Zhidkov, I.; Tsarev, S.A.; Luchkin, S.Y.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; et al. Intrinsic thermal decomposition pathways of lead halide perovskites APbX3. Sol. Energy Mater. Sol. Cells 2020, 213, 110559. [Google Scholar] [CrossRef]
- Zheng, J.; Yue, G.; Zhou, Z.A.; Li, H.; Hou, L.; Sun, C.; Li, X.; Kang, L.; Wang, N.; Zhao, Y.; et al. Phase transition induced thermal reversible luminescent of perovskite quantum dots fibers. Adv. Func. Mater. 2023, 33, 2300607. [Google Scholar] [CrossRef]
- Rizzo, A.; Lamberti, F.; Buonomo, M.; Wrachien, N.; Torto, L.; Lago, N.; Sansoni, S.; Pilot, R.; Prato, M.; Michieli, N.; et al. Understanding lead iodide perovskite hysteresis and degradation causes by extensive electrical characterization. Sol. Energy Mater. Sol. Cells 2019, 189, 43–52. [Google Scholar] [CrossRef]
- Leijtens, T.; Hoke, E.T.; Grancini, G.; Slotcavage, D.J.; Eperon, G.E.; Ball, J.M.; De Bastiani, M.; Bowring, A.R.; Martino, N.; Wojciechowski, K.; et al. Mapping electric field-induced switchable poling and structural degradation in hybrid lead halide perovskite thin films. Adv. Energy Mater. 2015, 5, 1614–6832. [Google Scholar] [CrossRef]
- Deng, X.; Wen, X.; Lau, C.F.J.; Young, T.; Yun, J.; Green, M.A.; Huang, S.; Ho-Baillie, A.W.Y. Electric field induced reversible and irreversible photoluminescence responses in methylammonium lead iodide perovskite. J. Mater. Chem. C 2016, 4, 9060–9068. [Google Scholar] [CrossRef]
- Bell, A.J. On the origin of the large piezoelectric effect in morphotropic phase boundary perovskite single crystals. Appl. Phys. Lett. 2000, 76, 109–111. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, M.; Fan, K.; Bao, C.; Xin, D.; Pan, Z.; Fei, L.; Huang, H.; Zhou, L.; Yao, K.; et al. Synergistic strain engineering of perovskite single crystals for highly stable and sensitive X-ray detectors with low-bias imaging and monitoring. Nat. Photonics 2022, 16, 575–581. [Google Scholar] [CrossRef]
- Luana, V.; Costales, A.; Pendas, A.M. Ions in crystals: The topology of the electron density in ionic materials. II. The cubic alkali halide perovskites. Phys. Rev. B 1997, 55, 4285–4297. [Google Scholar] [CrossRef]
- Lee, J.-W.; Tan, S.; Seok, S.I.; Yang, Y.; Park, N.-G. Rethinking the A cation in halide perovskites. Science 2022, 375, eabj1186. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Yao, H.; Zhu, W.; Hu, J.; Wu, C.; Wang, S.; Zhao, X.; Niu, X.; Ding, L.; Hao, F. Halogen engineering of 2d/3d tin halide perovskite for enhanced structural stability. Chem. Eng. J. 2023, 455, 140862. [Google Scholar] [CrossRef]
- Liang, Z.; Ni, L.; Zhang, Y.; Yuan, C.; Huang, L.; Yang, Y.; Xiao, Y. Effects of tellurium doping on environmental stability and luminous performance of CsPbBr3 quantum dots. ACS Omega 2022, 7, 21800–21807. [Google Scholar] [CrossRef]
- Zhao, Y.; Yavuz, I.; Wang, M.; Weber, M.H.; Xu, M.; Lee, J.-H.; Tan, S.; Huang, T.; Meng, D.; Wang, R.; et al. Suppressing ion migration in metal halide perovskite via interstitial doping with a trace amount of multivalent cations. Nat. Mater. 2022, 21, 1396–1402. [Google Scholar] [CrossRef]
- Lien, S.-Y.; Chen, Y.-H.; Chen, W.-R.; Liu, C.-H.; Huang, C.-J. Effect of growth temperature on the characteristics of cspbi3-quantum dots doped perovskite film. Molecules 2021, 26, 4439. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Ni, Z.; Xu, S.; Zhao, J.; Xiao, X.; Huang, J. Heterojunction structures for reduced noise in large-area and sensitive perovskite X-ray detectors. Sci. Adv. 2021, 7, 2375–2548. [Google Scholar] [CrossRef]
- Yan, J.; Gao, F.; Tian, Y.; Li, Y.; Gong, W.; Wang, S.; Zhu, H.; Li, L. Controllable perovskite single crystal heterojunction for stable self-powered photo-imaging and X-ray detection. Adv. Opt. Mater. 2022, 10, 2200449. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.-U.; Lee, D.Y.; Paik, M.J.; Na, H.; Lee, J.; Seok, S.I. Optimal interfacial engineering with different length of alkylammonium halide for efficient and stable perovskite solar cells. Adv. Energy Mater. 2019, 9, 1902740. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew. Chem. Int. Edit. 2014, 53, 11232–11235. [Google Scholar] [CrossRef]
- Zhou, N.; Shen, Y.; Li, L.; Tan, S.; Liu, N.; Zheng, G.; Chen, Q.; Zhou, H. Exploration of crystallization kinetics in quasi two-dimensional perovskite and high performance solar cells. J. Am. Chem. Soc. 2018, 140, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, S.A.; Madsen, M.V.; Dam, H.F.; Jorgensen, M.; Fell, C.J.; Anderson, K.E.; Duck, B.C.; Mescheloff, A.; Katz, E.A.; Elschner, A.; et al. Interlaboratory outdoor stability studies of flexible roll-to-roll coated organic photovoltaic modules: Stability over 10,000 h. Sol. Energy Mater. Sol. Cells 2013, 116, 187–196. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, Z.; Meng, X.; Yue, Y.; Ahmad, M.A.; Zhang, W.; Zhang, S.; Zhang, Y.; Liu, Z.; Chen, W. A review on encapsulation technology from organic light emitting diodes to organic and perovskite solar cells. Adv. Funct. Mater. 2021, 31, 2100151. [Google Scholar] [CrossRef]
- Hwang, I.; Jeong, I.; Lee, J.; Ko, M.J.; Yong, K. Enhancing stability of perovskite solar cells to moisture by the facile hydrophobic passivation. ACS Appl. Mater. Inter. 2015, 7, 17330–17336. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.-L.; Li, Y.; Yang, Y.-L.; Zhang, S.-L.; Zhao, J.; Wan, J.; Zhang, Z. Water-assistant ultrahigh fluorescence enhancement in perovskite polymer-encapsulated film for flexible X-ray scintillators. Chem. Eng. J. 2023, 452, 139132. [Google Scholar] [CrossRef]
- Bose, R.; Zheng, Y.; Guo, T.; Yin, J.; Hedhili, M.N.; Zhou, X.; Veyan, J.-F.; Gereige, I.; Al-Saggaf, A.; Gartstein, Y.N.; et al. Interface matters: Enhanced photoluminescence and long-term stability of zero-dimensional cesium lead bromide nanocrystals via gas-phase aluminum oxide encapsulation. ACS Appl. Mater. Inter. 2020, 12, 35598–35605. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, T.; Gao, W.; Niu, G.; Zhu, J.; Song, B.; Luo, J.; Pan, W.; Wu, H.; Zhang, M.; et al. Embedding Cs3Cu2I5 scintillators into anodic aluminum oxide matrix for high-resolution X-ray imaging. Adv. Opt. Mater. 2021, 9, 2101194. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, F. Advances in encapsulations for perovskite solar cells: From materials to applications. Solar RRL 2023, 7, 2201123. [Google Scholar] [CrossRef]
- Fang, H.-H.; Yang, J.; Tao, S.; Adjokatse, S.; Kamminga, M.E.; Ye, J.; Blake, G.R.; Even, J.; Loi, M.A. Unravelling light-induced degradation of layered perovskite crystals and design of efficient encapsulation for improved photostability. Adv. Funct. Mater. 2018, 28, 1800305. [Google Scholar] [CrossRef]
- Seitz, M.; Gant, P.; Castellanos-Gomez, A.; Prins, F. Long-term stabilization of two-dimensional perovskites by encapsulation with hexagonal boron nitride. Nanomaterials 2019, 9, 1120. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Xue, B.; Xiao, S.; Wang, X. Chemical Stability of Metal Halide Perovskite Detectors. Inorganics 2024, 12, 52. https://doi.org/10.3390/inorganics12020052
Zhang B, Xue B, Xiao S, Wang X. Chemical Stability of Metal Halide Perovskite Detectors. Inorganics. 2024; 12(2):52. https://doi.org/10.3390/inorganics12020052
Chicago/Turabian StyleZhang, Bin, Bin Xue, Shuang Xiao, and Xingzhu Wang. 2024. "Chemical Stability of Metal Halide Perovskite Detectors" Inorganics 12, no. 2: 52. https://doi.org/10.3390/inorganics12020052