Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity
Abstract
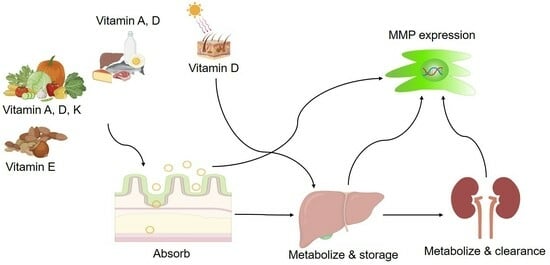
:1. Introduction
1.1. Fat-Soluble Vitamins (A, D, E, K)
1.2. Matrix Metalloproteinases-2 and -9
| MMP | Location | Substrate | Cell Types | Biological Function | Ref. |
|---|---|---|---|---|---|
| MMP-2 | ECM | Type IV Collagen, Gelatin, Fibronectin, Laminin, Aggrecan, Versican, Elastin | - | Matrix remodeling, angiogenesis, inflammation, invasion and metastasis | [28,47] |
| Cytoplasm | α-actinin, Tn1, Titin, MLC-1, Troponin I | Cardiomyocytes | Contractile dysfunction | [48,49] | |
| GSK-3β | Cardiomyoblast | Apoptosis | [50] | ||
| Talin | Platelets | Aggregation | [51,52] | ||
| Mitochondria | Hsp60, Cx43 | Retinal endothelial cells | Apoptosis | [53,54,55,56] | |
| IκB-α | Myoblastic cells | ||||
| Nucleus | PARP1, XRCC1 | Neurons | |||
| MMP-9 | ECM | Type IV Collagen, Gelatin, Fibronectin, Laminin, Aggrecan, Versican, Nidogen, Tenascin, CollagenX, Collagen III, Elastin | - | Matrix remodeling, angiogenesis, inflammation, invasion and metastasis | [28,47] |
| Cytoplasm | AMPKα | Leukocytes | Innate immunity | [57,58] | |
| MHC | Cardiomyocytes | ||||
| Mitochondria | Cx43, Hsp60, Hsp70 | Cardiomyocytes, Retinal cells | Apoptosis | [59,60] | |
| Nucleus | PARP1, XRCC1 | Neurons | Apoptosis | [55,61] | |
| Histone H3, Citrate synthase | Osteoclast, Cardiomyocytes |
2. Vitamin A
2.1. Transportation and Metabolism of Vitamin A
2.2. Cell Intake and Intracellular Activities
2.3. Homeostasis of Vitamin A and Related Diseases
2.4. Vitamin A in Cancer Treatment and MMP Regulation
3. Vitamin D
3.1. Transportation and Metabolism of Vitamin Ds
3.2. Cell Uptake and Intracellular Functions of Vitamin D
3.3. Functions of Vitamin D in the Human Body and Related Diseases
| Function | Activity | Related Disease | Ref. |
|---|---|---|---|
| Calcium homeostasis | Facilitation of calcium absorption in intestine and resorption in the renal tubules | Rickets | [6] |
| Osteomalacia | [5,149] | ||
| Immune regulation | Regulation of the expression and activity of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α, and IFN-γ) | Rheumatoid arthritis | [164] |
| Multiple sclerosis | [165,166] | ||
| Allergic diseases | [167] | ||
| Chronic diseases | [168] | ||
| Cardiovascular disease | [19,169] | ||
| Cancer | [170] | ||
| Cell growth regulation | Regulation of the expression of several genes involved in proliferation and differentiation | Cancers | [171] |
| Autoimmune diseases | [172] | ||
| MMP regulation | Direct and indirect regulation of the expression of MMP-2 and MMP-9 | Cancers | [173,174,175,176,177] |
| Liver fibrosis | [178] |
3.4. Vitamin D in Cancer Treatment and MMP Regulation
4. Vitamin E
4.1. Transportation and Homeostasis of Vitamin E
4.2. Functions of Vitamin E in the Human Body and Related Diseases
| Function | Activity | Related Diseases | Ref. |
|---|---|---|---|
| Antioxidant | Mitigates oxidative stress and counteracts free radicals | Cancer | [232] |
| Aging | [232,233] | ||
| Arthritis | [234,235] | ||
| Neuroprotection | Reduction in oxidative stress and aggregation of amyloid-β (Aβ) | AD | [215,236,237] |
| Parkinson’s disease (PD) | [238,239] | ||
| Cardiovascular health | Enhancement of nitric oxide synthase activity | Heart disease | [223] |
| Stroke | [240,241] | ||
| Skin health | Acting as a free radical scavenger | Skin damage | [222,242] |
| Skin cancer | [221] | ||
| MMP regulation | Regulating the expression of MMP-2 and MMP-9 through specific pathways, indirectly | Cancer | [207,208,209] |
| Inflammatory disorders | [20] |
4.3. Vitamin E for Treating Human Diseases and MMP Regulation
5. Vitamin K
5.1. Transportation and Homeostasis of Vitamin K in Human Body
5.2. Functions of Vitamin K in the Human Body and Related Diseases
| Function | Activity | Related Diseases | Ref. |
|---|---|---|---|
| Blood clotting | The accumulation of calcium in arteries and blood vessels Synthesis of Matrix Gla protein (MGP) Activation of blood clotting factors | Arterial calcification | [304,305] |
| Cardiovascular disease | [298] | ||
| Liver disease | [306,307] | ||
| Blood coagulation impairment | [308] | ||
| Neuroprotection | Sphingolipid metabolism modulation and Aβ clearance Regulation of Gas6 carboxylation and neuronal apoptosis | Alzheimer’s disease (AD) | [309] |
| Neurological disorders | [310] | ||
| Bone health | Fostering calcium absorption into bone | Osteoporosis | [15] |
| Fractures and weak bone structure | [311,312] | ||
| Wound healing | Enhanced cell proliferation and tissue repair | Diabetes Immune disorders | [313] [313,314,315] |
| MMPs regulation | Indirect influences on MMP-2 and MMP-9 expression | Cancer | [316,317] |
| Inflammatory disorders | [318,319] | ||
| Cardiovascular disease | [320] |
5.3. Vitamin K in Human Disease Treatment and MMP Regulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-Soluble Vitamins: Updated Review of Their Role and Orchestration in Human Nutrition throughout Life Cycle with Sex Differences. Nutr. Metab. 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Jialal, I. Biochemistry, Fat Soluble Vitamins; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Sommer, A.; Vyas, K.S. A Global Clinical View on Vitamin A and Carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of Retinoic Acid Receptors during Embryonic Development. Nucl. Recept. Signal. 2009, 7, e002. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Abe, E. Role of Vitamin D in Bone Resorption. J. Cell. Biochem. 1992, 49, 53–58. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, A.; Li, Y.; Han, X.; Wang, Q.; Liang, H. Vitamin E Supplementation Protects Erythrocyte Membranes from Oxidative Stress in Healthy Chinese Middle-Aged and Elderly People. Nutr. Res. 2012, 32, 328–334. [Google Scholar] [CrossRef]
- Tappel, A.L. Biological Antioxidant Protection against Lipid Peroxidation Damage. Am. J. Clin. Nutr. 1970, 23, 1137–1139. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Kujovská Krčmová, L.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L. Vitamin K—Sources, Physiological Role, Kinetics, Deficiency, Detection, Therapeutic Use, and Toxicity. Nutr. Rev. 2022, 80, 677–698. [Google Scholar] [CrossRef]
- Blomhoff, R.; Blomhoff, H.K. Overview of Retinoid Metabolism and Function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action (updated 31 December 2021). In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Alison, B., George, C., Emiliano, C., Wouter, W.H., Ketan, D., Kathleen, D., Johannes, H., et al., Eds.; DText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 757. [Google Scholar] [CrossRef]
- Zingg, J.M. Vitamin E: Regulatory Role on Signal Transduction. IUBMB Life 2019, 71, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Zhelev, Z.; Getsov, P.; Nikolova, B.; Aoki, I.; Higashi, T.; Bakalova, R. Vitamin K: Redox-Modulation, Prevention of Mitochondrial Dysfunction and Anticancer Effect. Redox. Biol. 2018, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.E.; Bak, M.J.; Narvaez, C.J. New Insights into Vitamin K Biology with Relevance to Cancer. Trends Mol. Med. 2022, 28, 864–881. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.A.; Lim, J.Y.; Kim, Y.; Jung, C.H.; Yoo, S.H.; Kim, Y. β-Carotene Inhibits Neuroblastoma Cell Invasion and Metastasis in Vitro and in Vivo by Decreasing Level of Hypoxia-Inducible Factor-1α. J. Nutr. Biochem. 2014, 25, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Yang, X.; Lu, M.; Hu, R.; Zhu, H.; Zhang, S.; Zhou, Q.; Chen, F.; Gui, S.; Wang, Y. All-Trans Retinoic Acid Inhibits Human Colorectal Cancer Cells RKO Migration via Downregulating Myosin Light Chain Kinase Expression through MAPK Signaling Pathway. Nutr. Cancer 2016, 68, 1225–1233. [Google Scholar] [CrossRef]
- Li, S.; Niu, G.; Dong, X.N.; Liu, Z.; Song, C.; Leng, H. Vitamin D Inhibits Activities of Metalloproteinase-9/-13 in Articular Cartilage In Vivo and In Vitro. J. Nutr. Sci. Vitaminol. 2019, 65, 107–112. [Google Scholar] [CrossRef]
- Khatami, P.G.; Soleimani, A.; Sharifi, N.; Aghadavod, E.; Asemi, Z. The Effects of High-Dose Vitamin E Supplementation on Biomarkers of Kidney Injury, Inflammation, and Oxidative Stress in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Lipidol. 2016, 10, 922–929. [Google Scholar] [CrossRef]
- Ide, Y.; Zhang, H.; Hamajima, H.; Kawaguchi, Y.; Eguchi, Y.; Mizuta, T.; Yamamoto, K.; Fujimoto, K.; Ozaki, I. Inhibition of Matrix Metalloproteinase Expression by Menatetrenone, a Vitamin K2 Analogue. Oncol. Rep. 2009, 22, 599–604. [Google Scholar]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar]
- Nguyen, Y.T.; Kim, N.; Lee, H.J. Metal Complexes as Promising Matrix Metalloproteinases Regulators. Int. J. Mol. Sci. 2023, 24, 1258. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 480558. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, C.K.; Santesso, M.R.; De Oliveira, G.S.N.; Ventura, T.M.D.S.; Doyama, J.T.; Zambuzzi, W.F.; Oliveira, R.C. De Updating the Role of Matrix Metalloproteinases in Mineralized Tissue and Related Diseases. J. Appl. Oral Sci. 2019, 27, e20180596. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Senn, N.; Riedl, R. Design and Structural Evolution of Matrix Metalloproteinase Inhibitors. Chemistry 2019, 25, 7960–7980. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Bischoff, R. Physiology and Pathophysiology of Matrix Metalloproteases. Amino Acids 2011, 41, 271. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; March, L.; Sambrook, P.N.; Jackson, C.J. Differential Regulation of Matrix Metalloproteinase 2 and Matrix Metalloproteinase 9 by Activated Protein C: Relevance to Inflammation in Rheumatoid Arthritis. Arthritis Rheum. 2007, 56, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Mattu, T.S.; Royle, L.; Langridge, J.; Wormald, M.R.; Van den Steen, P.E.; Van Damme, J.; Opdenakker, G.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M. O-Glycan Analysis of Natural Human Neutrophil Gelatinase B Using a Combination of Normal Phase-HPLC and Online Tandem Mass Spectrometry: Implications for the Domain Organization of the Enzyme. Biochemistry 2000, 39, 15695–15704. [Google Scholar] [CrossRef]
- Choi, H.; Kim, E.; Choi, J.Y.; Park, E.; Lee, H.J. Potent Therapeutic Targets for Treatment of Alzheimer’s Disease: Amyloid Degrading Enzymes. Bull. Korean Chem. Soc. 2021, 42, 1419–1429. [Google Scholar] [CrossRef]
- DeLeon-Pennell, K.Y.; Altara, R.; Yabluchanskiy, A.; Modesti, A.; Lindsey, M.L. The Circular Relationship between Matrix Metalloproteinase-9 and Inflammation Following Myocardial Infarction. IUBMB Life 2015, 67, 611–618. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The Role of TIMPs in Regulation of Extracellular Matrix Proteolysis. Matrix. Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Karadeniz, F.; Lee, S.G.; Oh, J.H.; Kim, J.A.; Kong, C.S. Inhibition of MMP-2 and MMP-9 Activities by Solvent-Partitioned Sargassum Horneri Extracts. Fish. Aquat. Sci. 2018, 21, 16. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The Tissue Inhibitors of Metalloproteinases (TIMPs): An Ancient Family with Structural and Functional Diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.X.; Li, H.X.; Chen, D.; Gao, Z.X.; Zheng, J.H. Changes in the Expression of MMP2, MMP9, and ColIV in Stromal Cells in Oral Squamous Tongue Cell Carcinoma: Relationships and Prognostic Implications. J. Exp. Clin. Cancer Res. 2012, 31, 90. [Google Scholar] [CrossRef]
- Blanco-Prieto, S.; Barcia-Castro, L.; Páez de la Cadena, M.; Rodríguez-Berrocal, F.J.; Vázquez-Iglesias, L.; Botana-Rial, M.I.; Fernández-Villar, A.; De Chiara, L. Relevance of Matrix Metalloproteases in Non-Small Cell Lung Cancer Diagnosis. BMC Cancer 2017, 17, 823. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The Relationship between MMP-2 and MMP-9 Expression Levels with Breast Cancer Incidence and Prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef]
- Long, H.; Zhou, B.; Jiang, F.G. Expression of MMP-2 and MMP-9 in Retinoblastoma and Their Significance. Int. J. Ophthalmol. 2011, 4, 489. [Google Scholar] [PubMed]
- Miao, C.; Liang, C.; Zhu, J.; Xu, A.; Zhao, K.; Hua, Y.; Zhang, J.; Chen, W.; Suo, C.; Zhang, C.; et al. Prognostic Role of Matrix Metalloproteinases in Bladder Carcinoma: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 32309–32321. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Q.; Liu, F.; Zhou, D. Prognostic Value of MMP-2 for Patients with Ovarian Epithelial Carcinoma: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2017, 295, 689–696. [Google Scholar] [CrossRef]
- Zeng, Z.S.; Cohen, A.M.; Guillem, J.G. Loss of Basement Membrane Type IV Collagen Is Associated with Increased Expression of Metalloproteinases 2 and 9 (MMP-2 and MMP-9) during Human Colorectal Tumorigenesis. Carcinogenesis 1999, 20, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Martins, J.M.; Rabelo-Santos, S.H.; Do Amaral Westin, M.C.; Zeferino, L.C. Tumoral and Stromal Expression of MMP-2, MMP-9, MMP-14, TIMP-1, TIMP-2, and VEGF-A in Cervical Cancer Patient Survival: A Competing Risk Analysis. BMC Cancer 2020, 20, 660. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Prasad, R.; Ansari, S.A.; Roy, A.; Mukherjee, A.; Sen, P. Matrix Metalloproteinase-2: A Key Regulator in Coagulation Proteases Mediated Human Breast Cancer Progression through Autocrine Signaling. Biomed. Pharmacother. 2018, 105, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Yan, L.; Du, W.; Zhang, M.; Chen, H.; Zhang, L.; Li, G.; Li, J.; Dong, Y.; et al. MMP-2 and MMP-9 Contribute to the Angiogenic Effect Produced by Hypoxia/15-HETE in Pulmonary Endothelial Cells. J. Mol. Cell. Cardiol. 2018, 121, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Myocardial Matrix Remodeling and the Matrix Metalloproteinases: Influence on Cardiac Form and Function. Physiol. Rev. 2007, 87, 1285–1342. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.M.; Fan, X.; Schulz, R. Cardiac Sarcomeric Proteins: Novel Intracellular Targets of Matrix Metalloproteinase-2 in Heart Disease. Trends Cardiovasc. Med. 2011, 21, 112–118. [Google Scholar] [CrossRef]
- Fan, X.; Hughes, B.G.; Ali, M.A.M.; Chan, B.Y.H.; Launier, K.; Schulz, R. Matrix Metalloproteinase-2 in Oncostatin M-Induced Sarcomere Degeneration in Cardiomyocytes. Am. J. Physiol. 2016, 311, 183–189. [Google Scholar] [CrossRef]
- Kandasamy, A.D.; Schulz, R. Glycogen Synthase Kinase-3β Is Activated by Matrix Metalloproteinase-2 Mediated Proteolysis in Cardiomyoblasts. Cardiovasc. Res. 2009, 83, 698–706. [Google Scholar] [CrossRef]
- Soslau, G.; Mason, C.; Lynch, S.; Benjamin, J.; Ashak, D.; Prakash, J.M.; Moore, A.; Bagsiyao, P.; Albert, T.; Mathew, L.M.; et al. Intracellular Matrix Metalloproteinase-2 (MMP-2) Regulates Human Platelet Activation via Hydrolysis of Talin. Thromb. Haemost. 2013, 111, 140–153. [Google Scholar]
- Larkin, C.M.; Hante, N.K.; Breen, E.P.; Tomaszewski, K.A.; Eisele, S.; Radomski, M.W.; Ryan, T.A.; Santos-Martinez, M.J. Role of Matrix Metalloproteinases 2 and 9, Toll-like Receptor 4 and Platelet-Leukocyte Aggregate Formation in Sepsis-Associated Thrombocytopenia. PLoS ONE 2018, 13, e0196478. [Google Scholar] [CrossRef]
- Mohammad, G.; Kowluru, R.A. Novel Role of Mitochondrial Matrix Metalloproteinase-2 in the Development of Diabetic Retinopathy. Investig. Ophthalmol. Vis Sci. 2011, 52, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Lovett, D.H.; Mahimkar, R.; Raffai, R.L.; Cape, L.; Maklashina, E.; Cecchini, G.; Karliner, J.S. A Novel Intracellular Isoform of Matrix Metalloproteinase-2 Induced by Oxidative Stress Activates Innate Immunity. PLoS ONE 2012, 7, e34177. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Poddar, R.; Thompson, J.F.; Rosenberg, G.A.; Yang, Y. Intranuclear Matrix Metalloproteinases Promote DNA Damage and Apoptosis Induced by Oxygen–Glucose Deprivation in Neurons. Neuroscience 2012, 220, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.J.; Chow, A.K.; Schulz, R.; Daniel, E.E. Matrix Metalloproteinase-2, Caveolins, Focal Adhesion Kinase and c-Kit in Cells of the Mouse Myocardium. J. Cell. Mol. Med. 2007, 11, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Amorosa, L.F.; Coyle, S.M.; Macor, M.A.; Lubitz, S.E.; Carson, J.L.; Birnbaum, M.J.; Lee, L.Y.; Haimovich, B. Proteolytic Cleavage of AMPKα and Intracellular MMP9 Expression Are Both Required for TLR4-Mediated MTORC1 Activation and HIF-1α Expression in Leukocytes. J. Immunol. 2015, 195, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Rouet-Benzineb, P.; Buhler, J.M.; Dreyfus, P.; Delcourt, A.; Dorent, R.; Perennec, J.; Crozatier, B.; Harf, A.; Lafuma, C. Altered Balance between Matrix Gelatinases (MMP-2 and MMP-9) and Their Tissue Inhibitors in Human Dilated Cardiomyopathy: Potential Role of MMP-9 in Myosin-Heavy Chain Degradation. Eur. J. Heart Fail. 1999, 1, 337–352. [Google Scholar] [CrossRef]
- De Bock, M.; Wang, N.; Decrock, E.; Bultynck, G.; Leybaert, L. Intracellular Cleavage of the Cx43 C-Terminal Domain by Matrix-Metalloproteases: A Novel Contributor to Inflammation? Mediat. Inflamm. 2015, 2015, 257471. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mohammad, G.; Dos Santos, J.M.; Zhong, Q. Abrogation of MMP-9 Gene Protects Against the Development of Retinopathy in Diabetic Mice by Preventing Mitochondrial Damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef]
- Kim, K.; Punj, V.; Kim, J.M.; Lee, S.; Ulmer, T.S.; Lu, W.; Rice, J.C.; An, W. MMP-9 Facilitates Selective Proteolysis of the Histone H3 Tail at Genes Necessary for Proficient Osteoclastogenesis. Genes Dev. 2016, 30, 208–219. [Google Scholar] [CrossRef]
- Radosinska, J.; Barancik, M.; Vrbjar, N. Heart Failure and Role of Circulating MMP-2 and MMP-9. Panminerva. Med. 2017, 59, 241–253. [Google Scholar] [CrossRef]
- Olejarz, W.; Łacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Ramiro, L.; Simats, A.; Hernández-Guillamon, M.; Delgado, P.; Bustamante, A.; Rosell, A. Matrix Metalloproteinases and ADAMs in Stroke. Cell. Mol. Life. Sci. 2019, 76, 3117–3140. [Google Scholar] [CrossRef] [PubMed]
- Terni, B.; Ferrer, I. Abnormal Expression and Distribution of MMP2 at Initial Stages of Alzheimer’s Disease-Related Pathology. J. Alzheimers Dis. 2015, 46, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.Y.; Jackson, C.J. Endogenous MMP-9 and Not MMP-2 Promotes Rheumatoid Synovial Fibroblast Survival, Inflammation and Cartilage Degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A and Provitamin A Carotenoids. In Present Knowledge in Nutrition; Marriott, B.P., Birt, D.F., Stalling, V.A., Yates, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–91. [Google Scholar]
- Ross, A. Vitamin A. In Modern Nutrition in Health and Disease; Ross, A.C., Caballero, B.H., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Wolters Kluwer Health Adis (ESP): Waltham, MA, USA, 2012; pp. 260–277. [Google Scholar]
- Nagao, A. Oxidative Conversion of Carotenoids to Retinoids and Other Products. J. Nutr. 2004, 134, 237S–240S. [Google Scholar] [CrossRef]
- Blomhoff, R. Vitamin A in Health and Disease; CRC Press: New York, NY, USA, 1994; pp. 1–30. [Google Scholar]
- Sommer, A.; West, K.P. (Eds.) Vitamin A Deficiency: Health, Survival, and Vision; Oxford University Express: New York, NY, USA, 1996; pp. 3–8. [Google Scholar]
- Dewett, D.; Lam-Kamath, K.; Poupault, C.; Khurana, H.; Rister, J. Mechanisms of Vitamin A Metabolism and Deficiency in the Mammalian and Fly Visual System. Dev. Biol. 2021, 476, 68–78. [Google Scholar] [CrossRef]
- Harrison, E.H.; Hussain, M.M. Mechanisms Involved in the Intestinal Digestion and Absorption of Dietary Vitamin A. J. Nutr. 2001, 131, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.D. Hepatic Uptake of Chylomicron Remnants. J. Lipid. Res. 1997, 38, 2173–2192. [Google Scholar] [CrossRef]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.C.M.A.; Blokzijl, H.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2018, 10, 29. [Google Scholar] [CrossRef]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A Metabolism: An Update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef]
- Green, M.H.; Green, J.B. Quantitative and Conceptual Contributions of Mathematical Modeling to Current Views on Vitamin A Metabolism, Biochemistry, and Nutrition. Adv. Food Nutr. Res. 1996, 40, 3–24. [Google Scholar] [PubMed]
- Van Bennekum, A.M.; Wei, S.; Gamble, M.V.; Vogel, S.; Piantedosi, R.; Gottesman, M.; Episkopoui, V.; Blaner, W.S. Biochemical Basis for Depressed Serum Retinol Levels in Transthyretin-Deficient Mice. J. Biol. Chem. 2001, 276, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Herschel Conaway, H.; Henning, P.; Lerner, U.H. Vitamin A Metabolism, Action, and Role in Skeletal Homeostasis. Endocr. Rev. 2013, 34, 766–797. [Google Scholar] [CrossRef] [PubMed]
- Bastien, J.; Rochette-Egly, C. Nuclear Retinoid Receptors and the Transcription of Retinoid-Target Genes. Gene 2004, 328, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Balmer, J.E.; Blomhoff, R. Gene Expression Regulation by Retinoic Acid. J. Lipid. Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M. Retinoid-Related Orphan Receptors (RORs): Critical Roles in Development, Immunity, Circadian Rhythm, and Cellular Metabolism. Nucl. Recept. Signal. 2009, 7, e003. [Google Scholar] [CrossRef]
- Cañón, E.; Cosgaya, J.M.; Scsucova, S.; Aranda, A. Rapid Effects of Retinoic Acid on CREB and ERK Phosphorylation in Neuronal Cells. Mol. Biol. Cell 2004, 15, 5583. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Biological Role of Matrix Metalloproteinases: A Critical Balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; Knutson, D. Vitamin A in Reproduction and Development. Nutrients 2011, 3, 385. [Google Scholar] [CrossRef]
- Wolf, G. The Discovery of the Visual Function of Vitamin A. J. Nutr. 2001, 131, 1647–1650. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Degos, L.; Wang, Z.Y. All Trans Retinoic Acid in Acute Promyelocytic Leukemia. Oncogene 2001, 20, 7140–7145. [Google Scholar] [CrossRef]
- Tallman, M.S.; Andersen, J.W.; Schiffer, C.A.; Appelbaum, F.R.; Feusner, J.H.; Woods, W.G.; Ogden, A.; Weinstein, H.; Shepherd, L.; Willman, C.; et al. All-Trans Retinoic Acid in Acute Promyelocytic Leukemia: Long-Term Outcome and Prognostic Factor Analysis from the North American Intergroup Protocol. Blood 2002, 100, 4298–4302. [Google Scholar] [CrossRef]
- Vu, H.T.; Hoang, T.X.; Kim, J.Y. All-Trans Retinoic Acid Enhances Matrix Metalloproteinase 2 Expression and Secretion in Human Myeloid Leukemia THP-1 Cells. Biomed. Res. Int. 2018, 2018, 5971080. [Google Scholar] [CrossRef]
- Wang, F.; Wang, N.; Gao, Y.; Zhou, Z.; Liu, W.; Pan, C.; Yin, P.; Yu, X.; Tang, M. β-Carotene Suppresses Osteoclastogenesis and Bone Resorption by Suppressing NF-ΚB Signaling Pathway. Life Sci. 2017, 174, 15–20. [Google Scholar] [CrossRef]
- De Jonge, E.A.L.; Kiefte-De Jong, J.C.; Campos-Obando, N.; Booij, L.; Franco, O.H.; Hofman, A.; Uitterlinden, A.G.; Rivadeneira, F.; Zillikens, M.C. Dietary Vitamin A Intake and Bone Health in the Elderly: The Rotterdam Study. Eur. J. Clin. Nutr. 2015, 69, 1360–1368. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Modulation of MMP-2 and MMP-9 Secretion by Cytokines, Inducers and Inhibitors in Human Glioblastoma T-98G Cells. Oncol. Rep. 2017, 37, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zheng, X.; Wu, X.; Wang, S.; Wang, Y.; Xing, F. All-Trans Retinoic Acid Reverses Epithelial-Mesenchymal Transition in Paclitaxel-Resistant Cells by Inhibiting Nuclear Factor Kappa B and Upregulating Gap Junctions. Cancer Sci. 2019, 110, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Yang, C.M.; Chen, J.Y.; Liao, J.W.; Hu, M.L. Alpha-Carotene Inhibits Metastasis in Lewis Lung Carcinoma in Vitro, and Suppresses Lung Metastasis and Tumor Growth in Combination with Taxol in Tumor Xenografted C57BL/6 Mice. J. Nutr. Biochem. 2015, 26, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Vijaya Kumar, P.; Giridharan, N.V.; Vajreswari, A. Vitamin A Improves Insulin Sensitivity by Increasing Insulin Receptor Phosphorylation through Protein Tyrosine Phosphatase 1B Regulation at Early Age in Obese Rats of WNIN/Ob Strain. Diabetes Obes. Metab. 2011, 13, 955–958. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A Signaling and Homeostasis in Obesity, Diabetes, and Metabolic Disorders. Pharmacol. Ther. 2019, 197, 153. [Google Scholar] [CrossRef]
- Hammouda, S.A.I.; Abd Al-Halim, O.A.F.; Mohamadin, A.M. Serum Levels of Some Micronutrients and Congenital Malformations: A Prospective Cohort Study in Healthy Saudi-Arabian First-Trimester Pregnant Women. Int. J. Vitam. Nutr. Res. 2013, 83, 346–354. [Google Scholar] [CrossRef]
- Chien, C.Y.; Lee, H.S.; Cho, C.H.H.; Lin, K.I.; Tosh, D.; Wu, R.R.; Mao, W.Y.; Shen, C.N. Maternal Vitamin A Deficiency during Pregnancy Affects Vascularized Islet Development. J. Nutr. Biochem. 2016, 36, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chang, L.; Rong, Z.; Liu, W. Retinoic Acid Diminished the Expression of MMP-2 in Hyperoxia-Exposed Premature Rat Lung Fibroblasts through Regulating Mitogen-Activated Protein Kinases. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2011, 31, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Dang, F.; Deng, C. β-Cryptoxanthin Induced Anti-Proliferation and Apoptosis by G0/G1 Arrest and AMPK Signal Inactivation in Gastric Cancer. Eur. J. Pharmacol. 2019, 859, 172528. [Google Scholar] [CrossRef] [PubMed]
- Burt Wolbach, S.; Howe, P.R. Tissue Changes Following Deprivation of Fat-Suluble A Vitamin. J. Exp. Med. 1925, 42, 753. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, H.K.; Smeland, E.B.; Erikstein, B.; Rasmussen, A.M.; Skrede, B.; Skjonsberg, C.; Blomhoff, R. Vitamin A Is a Key Regulator for Cell Growth, Cytokine Production, and Differentiation in Normal B Cells. J. Biol. Chem. 1992, 267, 23988–23992. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P. A Decade of Molecular Biology of Retinoic Acid Receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- Uray, I.P.; Dmitrovsky, E.; Brown, P.H. Retinoids and retinoids in cancer prevention: From laboratory to clinic. Semin. Oncol. 2016, 43, 49–64. [Google Scholar] [CrossRef]
- Gudas, L.J.; Wagner, J.A. Retinoids Regulate Stem Cell Differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A Challenging Paradigm of Cancer Management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, S.; Chen, J.; Zhao, S.; Cui, H.; Shirkoohi, R.; Kubo, N.; Kuge, Y.; Tamaki, N.; Nakagawa, K.; Hamada, J.; et al. All-Trans Retinoic Acid Enhances Murine Dendritic Cell Migration to Draining Lymph Nodes via the Balance of Matrix Metalloproteinases and Their Inhibitors. J. Immunol. 2007, 179, 4616–4625. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, M.; Saidijam, M.; Etemadi, K.; Najafi, R. All-Trans Retinoic Acid Enhances in Vitro Mesenchymal Stem Cells Migration by Targeting Matrix Metalloproteinases 2 and 9. Biotechnol. Lett. 2017, 39, 1263–1268. [Google Scholar] [CrossRef]
- Korzekwa, A.J.; Kononiuk, A.; Kordan, W.; Orzołek, A. Retinoic Acid Alters Metalloproteinase Action in Red Deer Antler Stem Cells. PLoS ONE 2023, 18, e0287782. [Google Scholar] [CrossRef] [PubMed]
- Chryssanthi, D.G.; Dedes, P.G.; Karamanos, N.K.; Cordopatis, P.; Lamari, F.N. Crocetin Inhibits Invasiveness of MDA-MB-231 Breast Cancer Cells via Downregulation of Matrix Metalloproteinases. Planta. Med. 2011, 77, 146–151. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Monterrey, J.; Rath, M.; Niedzwiecki, A. In Vitro Modulation of MMP-2 and MMP-9 in Adult Human Sarcoma Cell Lines by Cytokines, Inducers and Inhibitors. Int. J. Oncol. 2013, 43, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Tesniere, A.; Kroemer, G. Cancer despite Immunosurveillance: Immunoselection and Immunosubversion. Nat. Rev. Immunol. 2006, 6, 715–727. [Google Scholar] [CrossRef]
- Shurin, M.R.; Shurin, G.V.; Lokshin, A.; Yurkovetsky, Z.R.; Gutkin, D.W.; Chatta, G.; Zhong, H.; Han, B.; Ferris, R.L. Intratumoral Cytokines/Chemokines/Growth Factors and Tumor Infiltrating Dendritic Cells: Friends or Enemies? Cancer Metastasis Rev. 2006, 25, 333–356. [Google Scholar] [CrossRef]
- Ratzinger, G.; Stoitzner, P.; Ebner, S.; Lutz, M.B.; Layton, G.T.; Rainer, C.; Senior, R.M.; Shipley, J.M.; Fritsch, P.; Schuler, G.; et al. Matrix Metalloproteinases 9 and 2 Are Necessary for the Migration of Langerhans Cells and Dermal Dendritic Cells from Human and Murine Skin. J. Immunol. 2002, 168, 4361–4371. [Google Scholar] [CrossRef]
- Saito, D.; Imai, M.; Hasegawa, S.; Yamasaki, M.; Takahashi, N. A Splicing Factor Phosphorylated by Protein Kinase A Is Increased in HL60 Cells Treated with Retinoic Acid. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119142. [Google Scholar] [CrossRef]
- Roskoski, R. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumor Progression. Crit. Rev. Oncol. Hematol. 2007, 62, 179–213. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of Matrix Metalloproteinases in Cancer Progression and Their Pharmacological Targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A Millenium Perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef]
- Catharine, R.A.; Christine, T.L.; Ann, Y.L.; Heather, D.V.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press (US): Washington, DC, USA, 2011; pp. 3–10. [Google Scholar]
- Askew, F.A.; Bourdillon, R.B.; Bruce, H.M.; Jenkins, R.G.C.; Webster, T.A. The Distillation of Vitamin D. Proc. R. Soc. B 1930, 170, 76–90. [Google Scholar]
- Silva, M.C.; Furlanetto, T.W. Intestinal Absorption of Vitamin D: A Systematic Review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Cooke, N.E.; Haddad, J.G. Vitamin D Binding Protein (Gc-Globulin). Endocr. Rev. 1989, 10, 294–307. [Google Scholar] [CrossRef]
- Haddad, J.G.; Matsuoka, L.Y.; Hollis, B.W.; Hu, Y.Z.; Wortsman, J. Human Plasma Transport of Vitamin D after Its Endogenous Synthesis. J. Clin. Investig. 1993, 91, 2552. [Google Scholar] [CrossRef]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A.G. Vitamin D3 Distribution and Status in the Body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef]
- Bikle, D.D. Extra Renal Synthesis of 1,25-Dihydroxyvitamin D and Its Health Implications. Clin. Rev. Bone Miner. Metab. 2009, 7, 114–125. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal Expression of 25-Hydroxyvitamin d(3)-1 Alpha-Hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar]
- Panda, D.K.; Kawas, S.A.; Seldin, M.F.; Hendy, G.N.; Goltzman, D. 25-Hydroxyvitamin D 1alpha-Hydroxylase: Structure of the Mouse Gene, Chromosomal Assignment, and Developmental Expression. J. Bone Miner. Res. 2001, 16, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Nemanic, M.K.; Whitney, J.O.; Elias, P.W. Neonatal Human Foreskin Keratinocytes Produce 1,25-Dihydroxyvitamin D3. Biochemistry 1986, 25, 1545–1548. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020, 4, bvz038. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.K.; Lin, D.; Zhang, M.Y.H.; Bikle, D.D.; Shackleton, C.H.L.; Miller, W.L.; Portale, A.A. Cloning of Human 25-Hydroxyvitamin D-1 Alpha-Hydroxylase and Mutations Causing Vitamin D-Dependent Rickets Type 1. Mol. Endocrinol. 1997, 11, 1961–1970. [Google Scholar] [PubMed]
- Bikle, D.D.; Halloran, B.P.; Gee, E.; Ryzen, E.; Haddad, J.G. Free 25-Hydroxyvitamin D Levels Are Normal in Subjects with Liver Disease and Reduced Total 25-Hydroxyvitamin D Levels. J. Clin. Investig. 1986, 78, 748–752. [Google Scholar] [CrossRef]
- Madden, K.; Feldman, H.A.; Chun, R.F.; Smith, E.M.; Sullivan, R.M.; Agan, A.A.; Keisling, S.M.; Panoskaltsis-Mortari, A.; Randolph, A.G. Critically Ill Children Have Low Vitamin D-Binding Protein, Influencing Bioavailability of Vitamin D. Ann. Am. Thorac. Soc. 2015, 12, 1654–1661. [Google Scholar] [CrossRef]
- Safadi, F.F.; Thornton, P.; Magiera, H.; Hollis, B.W.; Gentile, M.; Haddad, J.G.; Liebhaber, S.A.; Cooke, N.E. Osteopathy and Resistance to Vitamin D Toxicity in Mice Null for Vitamin D Binding Protein. J. Clin. Investig. 1999, 103, 239–251. [Google Scholar] [CrossRef]
- Zella, L.A.; Shevde, N.K.; Hollis, B.W.; Cooke, N.E.; Pike, J.W. Vitamin D-Binding Protein Influences Total Circulating Levels of 1,25-Dihydroxyvitamin D3 but Does Not Directly Modulate the Bioactive Levels of the Hormone in Vivo. Endocrinology 2008, 149, 3656–3667. [Google Scholar] [CrossRef]
- Henderson, C.M.; Fink, S.L.; Bassyouni, H.; Argiropoulos, B.; Brown, L.; Laha, T.J.; Jackson, K.J.; Lewkonia, R.; Ferreira, P.; Hoofnagle, A.N.; et al. Vitamin D–Binding Protein Deficiency and Homozygous Deletion of the GC Gene. N. Engl. J. Med. 2019, 380, 1150–1157. [Google Scholar] [CrossRef]
- Aita, R.; Aldea, D.; Hassan, S.; Hur, J.; Pellon-Cardenas, O.; Cohen, E.; Chen, L.; Shroyer, N.; Christakos, S.; Verzi, M.P.; et al. Genomic Analysis of 1,25-Dihydroxyvitamin D3 Action in Mouse Intestine Reveals Compartment and Segment-Specific Gene Regulatory Effects. J. Biol. Chem. 2022, 298, 102213. [Google Scholar] [CrossRef]
- Norman, A.W. Vitamin D Receptor: New Assignments for an Already Busy Receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Yoshimoto, Y.; Norman, A.W. Calcium Transport in Perfused Duodena from Normal Chicks: Enhancement within Fourteen Minutes of Exposure to 1,25-Dihydroxyvitamin D3. Endocrinology 1984, 115, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- De Boland, A.R.; Norman, A.W. Influx of Extracellular Calcium Mediates 1,25-Dihydroxyvitamin D3-Dependent Transcaltachia (the Rapid Stimulation of Duodenal Ca2+ Transport). Endocrinology 1990, 127, 2475–2480. [Google Scholar] [CrossRef]
- De Boland, A.R.; Nemere, I.; Norman, A.W. Ca2+-Channel Agonist Bay K8644 Mimics 1,25(OH)2-Vitamin D3 Rapid Enhancement of Ca2+ Transport in Chick Perfused Duodenum. Biochem. Biophys. Res. Commun. 1990, 166, 217–222. [Google Scholar] [CrossRef]
- Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D and Regulation of Vascular Cell Function. Am. J. Physiol. 2018, 314, H753–H765. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D Receptor Inhibits Nuclear Factor ΚB Activation by Interacting with IκB Kinase β Protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef]
- Lange, C.M.; Gouttenoire, J.; Duong, F.H.T.; Morikawa, K.; Heim, M.H.; Moradpour, D. Vitamin D Receptor and Jak-STAT Signaling Crosstalk Results in Calcitriol-Mediated Increase of Hepatocellular Response to IFN-α. J. Immunol. 2014, 192, 6037–6044. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, W.; Kong, J.; Zheng, W.; Szeto, F.L.; Wong, K.E.; Cohen, R.; Klopot, A.; Zhang, Z.; Yan, C.L. 1,25-Dihydroxyvitamin D3 Suppresses Renin Gene Transcription by Blocking the Activity of the Cyclic AMP Response Element in the Renin Gene Promoter. J. Biol. Chem. 2007, 282, 29821–29830. [Google Scholar] [CrossRef]
- Becker-Weimann, S.; Xiong, G.; Furuta, S.; Han, J.; Kuhn, I.; Akavia, U.D.; Pe’er, D.; Bissell, M.J.; Xu, R. NFkB Disrupts Tissue Polarity in 3D by Preventing Integration of Microenvironmental Signals. Oncotarget 2013, 4, 2010–2020. [Google Scholar] [CrossRef]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. North Am. 2017, 46, 815. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Martin, T.J. Role of Osteoblasts in Hormonal Control of Bone Resorption—A Hypothesis. Calcif. Tissue Int. 1981, 33, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guo, J.; Xie, W.; Yuan, L.; Sheng, X. The Role of Vitamin D in Ovarian Cancer: Epidemiology, Molecular Mechanism and Prevention. J. Ovarian. Res. 2018, 11, 71. [Google Scholar] [CrossRef]
- Rebelos, E.; Tentolouris, N.; Jude, E. The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation. Drugs 2023, 83, 665. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The Role of Vitamin D in Reducing Cancer Risk and Progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Extraskeletal Actions of Vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29. [Google Scholar] [CrossRef]
- Pettifor, J.M.; Bikle, D.D.; Cavaleros, M.; Zachen, D.; Kamdar, M.C.; Ross, F.P. Serum Levels of Free 1,25-Dihydroxyvitamin D in Vitamin D Toxicity. Ann. Intern. Med. 1995, 122, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lewandowska, U. Vitamin D, the Vitamin D Receptor, Calcitriol Analogues and Their Link with Ocular Diseases. Nutrients 2022, 14, 2353. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbanska, M.; Lukaszkiewicz, J.; Pludowski, P.; Jones, G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Lanham-New, S.; Vieth, R.; Heaney, R. Vitamin D2 and Vitamin D3 Comparisons: Fundamentally Flawed Study Methodology. Am. J. Clin. Nutr. 2010, 92, 999. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of Vitamin D2 and Vitamin D3 Supplementation in Raising Serum 25-Hydroxyvitamin D Status: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D Deficiency as a Public Health Issue: Using Vitamin D2 or Vitamin D3 in Future Fortification Strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Wilson, L.R.; Hart, K.; Johnsen, S.; De Lusignan, S.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Elliott, R.; et al. Daily Supplementation with 15 Μg Vitamin D2 Compared with Vitamin D3 to Increase Wintertime 25-Hydroxyvitamin D Status in Healthy South Asian and White European Women: A 12-Wk Randomized, Placebo-Controlled Food-Fortification Trial. Am. J. Clin. Nutr. 2017, 106, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Durrant, L.R.; Bucca, G.; Hesketh, A.; Möller-Levet, C.; Tripkovic, L.; Wu, H.; Hart, K.H.; Mathers, J.C.; Elliott, R.M.; Lanham-New, S.A.; et al. Vitamins D2 and D3 Have Overlapping But Different Effects on the Human Immune System Revealed Through Analysis of the Blood Transcriptome. Front. Immunol. 2022, 13, 790444. [Google Scholar] [CrossRef]
- Zittermann, A.; Pilz, S.; Berthold, H.K. Serum 25-Hydroxyvitamin D Response to Vitamin D Supplementation in Infants: A Systematic Review and Meta-Analysis of Clinical Intervention Trials. Eur. J. Nutr. 2020, 59, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between Vitamin D Supplementation and Mortality: Systematic Review and Meta-Analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef]
- Meena, N.; Chawla, S.P.S.; Garg, R.; Batta, A.; Kaur, S. Assessment of Vitamin D in Rheumatoid Arthritis and Its Correlation with Disease Activity. J. Nat. Sci. Biol. Med. 2018, 9, 54. [Google Scholar]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59. [Google Scholar] [CrossRef]
- Mahon, B.D.; Gordon, S.A.; Cruz, J.; Cosman, F.; Cantorna, M.T. Cytokine Profile in Patients with Multiple Sclerosis Following Vitamin D Supplementation. J. Neuroimmunol. 2003, 134, 128–132. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Busa, P.; Huang, N.; Kuthati, Y.; Wong, C.S. Vitamin D Reduces Pain and Cartilage Destruction in Knee Osteoarthritis Animals through Inhibiting the Matrix Metalloprotease (MMPs) Expression. Heliyon 2023, 9, e15268. [Google Scholar] [CrossRef] [PubMed]
- Gatera, V.A.; Lesmana, R.; Musfiroh, I.; Judistiani, R.T.D.; Setiabudiawan, B.; Abdulah, R. Vitamin D Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in A549 Cells by Downregulating Inflammatory Cytokines. Med. Sci. Monit. Basic Res. 2021, 27, e931481. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barral, A.; Bustamante-Madrid, P.; Ferrer-Mayorga, G.; Barbáchano, A.; Larriba, M.J.; Muñoz, A. Vitamin D Effects on Cell Differentiation and Stemness in Cancer. Cancers 2020, 12, 2413. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Bajbouj, K.; Al-Ali, A.; Shafarin, J.; Sahnoon, L.; Sawan, A.; Shehada, A.; Elkhalifa, W.; Saber-Ayad, M.; Muhammad, J.S.; Elmoselhi, A.B.; et al. Vitamin D Exerts Significant Antitumor Effects by Suppressing Vasculogenic Mimicry in Breast Cancer Cells. Front. Oncol. 2022, 12, 918340. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, F.; Yang, W.; Shi, W.; Wan, J.; Li, J.; Pan, J.; Wang, P.; Qiu, J.; Zhang, Z.; et al. Effect of 1α,25(OH)2D3-Treated M1 and M2 Macrophages on Cell Proliferation and Migration Ability in Ovarian Cancer. Nutr. Cancer 2022, 74, 2632–2643. [Google Scholar] [CrossRef]
- Baek, M.S.; Yoon, D.S.; Park, J.S.; Yoon, B.W.; Oh, B.S.; Park, J.; Kim, H.J. Vitamin D Inhibits Expression and Activity of Matrix Metalloproteinase in Human Lung Fibroblasts (HFL-1) Cells. Tuberc. Respir. Dis. 2014, 77, 73–80. [Google Scholar]
- Zhao, Y.; Wang, H.; Li, X.; Cao, M.; Lu, H.; Meng, Q.; Pang, H.; Li, H.; Nadolny, C.; Dong, X.; et al. Ang II-AT1R Increases Cell Migration through PI3K/AKT and NF-ΚB Pathways in Breast Cancer. J. Cell. Physiol. 2014, 229, 1855–1862. [Google Scholar] [CrossRef]
- Mon, N.N.; Senga, T.; Ito, S. Interleukin-1β Activates Focal Adhesion Kinase and Src to Induce Matrix Metalloproteinase-9 Production and Invasion of MCF-7 Breast Cancer Cells. Oncol. Lett. 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Gong, J.; Gong, H.Y.; Liu, Y.; Tao, X.L.; Zhang, H. Calcipotriol Attenuates Liver Fibrosis through the Inhibition of Vitamin D Receptor-Mediated NF-ΚB Signaling Pathway. Bioengineered 2022, 13, 2658–2672. [Google Scholar] [CrossRef]
- Garland, C.; Barrett-Connor, E.; Rossof, A.H.; Shekelle, R.B.; Criqui, M.H.; Paul, O. Dietary Vitamin D and Calcium and Risk of Colorectal Cancer: A 19-Year Prospective Study in Men. Lancet 1985, 1, 307–309. [Google Scholar] [CrossRef]
- Bostick, R.M.; Potter, J.D.; Sellers, T.A.; Mckenzie, D.R.; Kushi, L.H.; Folsom, A.R. Relation of Calcium, Vitamin D, and Dairy Food Intake to Incidence of Colon Cancer among Older Women. The Iowa Women’s Health Study. Am. J. Epidemiol. 1993, 137, 1302–1317. [Google Scholar] [CrossRef]
- Kearney, J.; Giovannucci, E.; Rimm, E.B.; Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Wing, A.; Kampman, E.; Willett, W.C. Calcium, Vitamin D, and Dairy Foods and the Occurrence of Colon Cancer in Men. Am. J. Epidemiol. 1996, 143, 907–917. [Google Scholar] [PubMed]
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (united State). Cancer Cause Control 2005, 16, 83–95. [Google Scholar]
- Yuan, C.; Qian, Z.R.; Babic, A.; Morales-Oyarvide, V.; Rubinson, D.A.; Kraft, P.; Ng, K.; Bao, Y.; Giovannucci, E.L.; Ogino, S.; et al. Prediagnostic Plasma 25-Hydroxyvitamin D and Pancreatic Cancer Survival. J. Clin. Oncol. 2016, 34, 2899–2905. [Google Scholar] [CrossRef]
- Rasmussen, L.S.; Yilmaz, M.K.; Falkmer, U.G.; Poulsen, L.; Bøgsted, M.; Christensen, H.S.; Bojesen, S.E.; Jensen, B.V.; Chen, I.M.; Johansen, A.Z.; et al. Pre-Treatment Serum Vitamin D Deficiency Is Associated with Increased Inflammatory Biomarkers and Short Overall Survival in Patients with Pancreatic Cancer. Eur. J. Cancer 2021, 144, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Barry, E.L.; Mott, L.A.; Rees, J.R.; Sandler, R.S.; Snover, D.C.; Bostick, R.M.; Ivanova, A.; Cole, B.F.; Ahnen, D.J.; et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N. Engl. J. Med. 2015, 373, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Reins, R.Y.; Baidouri, H.; McDermott, A.M. Vitamin D Activation and Function in Human Corneal Epithelial Cells During TLR-Induced Inflammation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7715–7727. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Chiang, E.P.I.; Syu, J.N.; Chao, C.Y.; Lin, H.Y.; Lin, C.C.; Yang, M.D.; Tsai, S.Y.; Tang, F.Y. Treatment of 13-Cis Retinoic Acid and 1,25-Dihydroxyvitamin D3 Inhibits TNF-Alpha-Mediated Expression of MMP-9 Protein and Cell Invasion through the Suppression of JNK Pathway and MicroRNA 221 in Human Pancreatic Adenocarcinoma Cancer Cells. PLoS ONE 2021, 16, e0247550. [Google Scholar] [CrossRef]
- Lim, K.; Molostvov, G.; Lubczanska, M.; Fletcher, S.; Bland, R.; Hiemstra, T.F.; Zehnder, D. Impaired Arterial Vitamin D Signaling Occurs in the Development of Vascular Calcification. PLoS ONE 2020, 15, e0241976. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.F. History of Vitamin E in Infant Nutrition. Am. J. Clin. Nutr. 1987, 46, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- El Hadi, H.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef]
- Bjørneboe, A.; Bjørneboe, G.E.A.; Drevon, C.A. Serum Half-Life, Distribution, Hepatic Uptake and Biliary Excretion of α-Tocopherol in Rats. Biochim. Biophys. Acta 1987, 921, 175–181. [Google Scholar] [CrossRef]
- Rigotti, A. Absorption, Transport, and Tissue Delivery of Vitamin E. Mol. Aspects. Med. 2007, 28, 423–436. [Google Scholar] [CrossRef]
- Traber, M.G.; Kayden, H.J. Tocopherol Distribution and Intracellular Localization in Human Adipose Tissue. Am. J. Clin. Nutr. 1987, 46, 488–495. [Google Scholar] [CrossRef]
- Kiyose, C. Absorption, Transportation, and Distribution of Vitamin E Homologs. Free Radic. Biol. Med. 2021, 177, 226–237. [Google Scholar] [CrossRef]
- Jiang, Q. Metabolism of Natural Forms of Vitamin E and Biological Actions of Vitamin E Metabolites. Free Radic. Biol. Med. 2022, 179, 375–387. [Google Scholar] [CrossRef]
- Martens, L.G.; Luo, J.; Meulmeester, F.L.; Ashrafi, N.; van Eekelen, E.W.; de Mutsert, R.; Mook-Kanamori, D.O.; Rosendaal, F.R.; van Dijk, K.W.; Mills, K.; et al. Associations between Lifestyle Factors and Vitamin E Metabolites in the General Population. Antioxidants 2020, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Morley, S.; Manor, D. Hepatic α-Tocopherol Transfer Protein: Ligand-Induced Protection from Proteasomal Degradation. Biochemistry 2010, 49, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Baroni, M.; Mangialasche, F.; Mecocci, P. Vitamin E Family: Role in the Pathogenesis and Treatment of Alzheimer’s Disease. Alzheimers Dement. 2016, 2, 182–191. [Google Scholar] [CrossRef]
- Yap, S.P.; Yuen, K.H.; Wong, J.W. Pharmacokinetics and Bioavailability of α-, γ- and δ-Tocotrienols under Different Food Status. J. Pharm. Pharmacol. 2010, 53, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.B.; Praticò, D. Vitamin E in Aging, Dementia, and Alzheimer’s Disease. BioFactors 2012, 38, 90–97. [Google Scholar] [CrossRef]
- O’Byrne, D.; Grundy, S.; Packer, L.; Devaraj, S.; Baldenius, K.; Hoppe, P.P.; Kraemer, K.; Jialal, I.; Traber, M.G. Studies of LDL Oxidation Following α-, γ-, or δ-Tocotrienyl Acetate Supplementation of Hypercholesterolemic Humans. Free Radic. Biol. Med. 2000, 29, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Vinayagamoorthi, R.; Bobby, Z.; Sridhar, M.G. Antioxidants Preserve Redox Balance and Inhibit C-Jun-N-Terminal Kinase Pathway While Improving Insulin Signaling in Fat-Fed Rats: Evidence for the Role of Oxidative Stress on IRS-1 Serine Phosphorylation and Insulin Resistance. J. Endocrinol. 2008, 197, 287–296. [Google Scholar] [CrossRef] [PubMed]
- De Nigris, F.; Franconi, F.; Maida, I.; Palumbo, G.; Anania, V.; Napoli, C. Modulation by α- and γ-Tocopherol and Oxidized Low-Density Lipoprotein of Apoptotic Signaling in Human Coronary Smooth Muscle Cells. Biochem. Pharmacol. 2000, 59, 1477–1487. [Google Scholar] [CrossRef]
- Kwang, S.A.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-Tocotrienol Inhibits Nuclear Factor-ΚB Signaling Pathway through Inhibition of Receptor-Interacting Protein and TAK1 Leading to Suppression of Antiapoptotic Gene Products and Potentiation of Apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar]
- Sun, W.; Wang, Q.; Chen, B.; Liu, J.; Liu, H.; Xu, W. γ-Tocotrienol-Induced Apoptosis in Human Gastric Cancer SGC-7901 Cells Is Associated with a Suppression in Mitogen-Activated Protein Kinase Signalling. Br. J. Nutr. 2008, 99, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Sylvester, P.W. γ-Tocotrienol Inhibits Neoplastic Mammary Epithelial Cell Proliferation by Decreasing Akt and Nuclear Factor ΚB Activity. Exp. Biol. Med. 2005, 230, 235–241. [Google Scholar] [CrossRef]
- Samant, G.V.; Sylvester, P.W. γ-Tocotrienol Inhibits ErbB3-Dependent PI3K/Akt Mitogenic Signalling in Neoplastic Mammary Epithelial Cells. Cell Prolif. 2006, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, P.W.; McIntyre, B.S.; Gapor, A.; Briski, K.P. Vitamin E Inhibition of Normal Mammary Epithelial Cell Growth Is Associated with a Reduction in Protein Kinase Cα Activation. Cell Prolif. 2001, 34, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Appathurai, A.; Yeoh, H.L.; Driscoll, K.; Faisal, W. Vitamin E in Cancer Treatment: A Review of Clinical Applications in Randomized Control Trials. Nutrients 2022, 14, 4329. [Google Scholar] [CrossRef]
- Bowman, B.A.B.; Russel, R. Present Knowledge in Nutrition, 9th ed.; Intl Life Science Inst: Washington, DC, USA, 2006; Volume 1, pp. 269–277. [Google Scholar]
- Dutta, S.K.; Bustin, M.P.; Russell, R.M.; Costa, B.S. Deficiency of Fat-Soluble Vitamins in Treated Patients with Pancreatic Insufficiency. Ann. Intern. Med. 1982, 97, 549–552. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Alias, D.; Ruiz-Tovar, J.; Moreno, A.; Manso, B.; Diaz, G.; Duran, M.; Garcia-Olmo, D. Effect of Subcutaneous Sterile Vitamin E Ointment on Incisional Surgical Site Infection after Elective Laparoscopic Colorectal Cancer Surgery. Surg. Infect. 2017, 18, 287–292. [Google Scholar] [CrossRef]
- Cardenas, E.; Ghosh, R. Vitamin E: A Dark Horse at the Crossroad of Cancer Management. Biochem. Pharmacol. 2013, 86, 845–852. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E and Metabolites—Regulation of Cancer Cell Death and Underlying Mechanisms. IUBMB Life 2019, 71, 495–506. [Google Scholar] [CrossRef]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E Function and Requirements in Relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef]
- Vitamin E—Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 7 October 2023).
- Zingg, J.M. Vitamin E: An Overview of Major Research Directions. Mol. Asp. Med. 2007, 28, 400–422. [Google Scholar] [CrossRef]
- Keen, M.; Hassan, I. Vitamin E in Dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Hong, C.G.; Florida, E.; Li, H.; Parel, P.M.; Mehta, N.N.; Sorokin, A.V. Oxidized Low-Density Lipoprotein Associates with Cardiovascular Disease by a Vicious Cycle of Atherosclerosis and Inflammation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2023, 9, 1023651. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the Prevention of Cardiovascular Disease in Men: The Physicians’ Health Study II Randomized Controlled Trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Ding, Z.; Saldeen, T.; Mehta, J.L. Tocopherols in the Prevention and Treatment of Atherosclerosis and Related Cardiovascular Disease. Clin. Cardiol. 2015, 38, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E Inadequacy in Humans: Causes and Consequences. Adv. Nutr. 2014, 5, 503–514. [Google Scholar] [CrossRef]
- Sokol, R.J.; Bove, K.E.; Heubi, J.E.; Iannaccone, S.T. Vitamin E Deficiency during Chronic Childhood Cholestasis: Presence of Sural Nerve Lesion Prior to 2½ Years of Age. J. Pediatr. 1983, 103, 197–204. [Google Scholar] [CrossRef]
- Kohlschutter, A.; Finckh, B.; Nickel, M.; Bley, A.; Hübner, C. First Recognized Patient with Genetic Vitamin e Deficiency Stable after 36 Years of Controlled Supplement Therapy. Neurodegener. Dis. 2020, 20, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Saldeen, T.; Romeo, F.; Mehta, J.L. Relative Effects of α- and γ-Tocopherol on Low-Density Lipoprotein Oxidation and Superoxide Dismutase and Nitric Oxide Synthase Activity and Protein Expression in Rats. J. Cardiovasc. Pharmacol. Ther. 1999, 4, 219–226. [Google Scholar] [PubMed]
- Colombo, M.L. An Update on Vitamin E, Tocopherol and Tocotrienol-Perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef]
- Kono, N.; Ohto, U.; Hiramatsu, T.; Urabe, M.; Uchida, Y.; Satow, Y.; Arai, H. Impaired α-TTP-PIPs Interaction Underlies Familial Vitamin E Deficiency. Science 2013, 340, 1106–1110. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Chin, K.Y.; Ima-Nirwana, S. The Role of Vitamin E in Preventing and Treating Osteoarthritis—A Review of the Current Evidence. Front. Pharmacol. 2018, 9, 946. [Google Scholar] [CrossRef]
- Brand, C.; Snaddon, J.; Bailey, M.; Cicuttini, F. Vitamin E Is Ineffective for Symptomatic Relief of Knee Osteoarthritis: A Six Month Double Blind, Randomised, Placebo Controlled Study. Ann. Rheum. Dis. 2001, 60, 946–949. [Google Scholar] [CrossRef]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s Disease: What Do We Know so Far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Alwardat, M.; Salimei, P.S.; Mercuri, N.B.; Pierantozzi, M.; et al. Dietary Vitamin E as a Protective Factor for Parkinson’s Disease: Clinical and Experimental Evidence. Front. Neurol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Schürks, M.; Glynn, R.J.; Rist, P.M.; Tzourio, C.; Kurth, T. Effects of Vitamin E on Stroke Subtypes: Meta-Analysis of Randomised Controlled Trials. BMJ 2010, 341, 1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant Effects of Vitamins C and E Are Associated With Altered Activation of Vascular NADPH Oxidase and Superoxide Dismutase in Stroke-Prone SHR. Hypertension 2001, 38, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E.; Clive, J.; Combs, G.F.; Commisso, J.; Keen, C.L.; Nakamura, C.L. Effects of Topical and Oral Vitamin E on Pigmentation and Skin Cancer Induced by Ultraviolet Irradiation in Skh:2 Hairless Mice. Nutr. Cancer 2000, 38, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Christen, S.; Woodall, A.A.; Shigenaga, M.K.; Southwell-Keely, P.T.; Duncan, M.W.; Ames, B.N. γ-Tocopherol Traps Mutagenic Electrophiles Such as NOx and Complements α-Tocopherol: Physiological Implications. Proc. Natl. Acad. Sci. USA 1997, 94, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S.; Schendel, K.; Yong, Q.D.; Barrera, S.L.; Spitz, M.R.; Forman, M.R. Dietary α-, β-, γ-, and δ-Tocopherols in Lung Cancer Risk. Int. J. Cancer. 2008, 123, 1173–1180. [Google Scholar] [CrossRef]
- Lee, I.M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.A.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the Primary Prevention of Cardiovascular Disease and Cancer: The Women’s Health Study: A Randomized Controlled Trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef]
- Sylvester, P.W. Vitamin E and Apoptosis. Vitam. Horm. 2007, 76, 329–356. [Google Scholar]
- Yu, W.; Simmons-Menchaca, M.; Gapor, A.; Sanders, B.G.; Kline, K. Induction of Apoptosis in Human Breast Cancer Cells by Tocopherols and Tocotrienols. Nutr. Cancer 1999, 33, 26–32. [Google Scholar] [CrossRef]
- Gohi, S.H.; Hew, N.F.; Norhanom, A.W.; Yadav, M. Inhibition of tumour promotion by various palm-oil tocotrienols. Int. J. Cancer 1994, 57, 529–531. [Google Scholar] [CrossRef]
- Rickmann, M.; Vaquero, E.C.; Malagelada, J.R.; Molero, X. Tocotrienols Induce Apoptosis and Autophagy in Rat Pancreatic Stellate Cells Through the Mitochondrial Death Pathway. Gastroenterology 2007, 132, 2518–2532. [Google Scholar] [CrossRef]
- Guthrie, N.; Gapor, A.; Chambers, A.F.; Carroll, K.K. Inhibition of Proliferation of Estrogen Receptor–Negative MDA-MB-435 and –Positive MCF-7 Human Breast Cancer Cells by Palm Oil Tocotrienols and Tamoxifen, Alone and in Combination. J. Nutr. 1997, 127, 544S–548S. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Effect of Tocotrienols on the Growth of a Human Breast Cancer Cell Line in Culture. Lipids 1995, 30, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Wang, Q.; Li, Y.; Sun, W.G.; Liu, J.R.; Yang, Y.M.; Xu, W.L.; Sun, X.R.; Chen, B.Q. Inhibitory Effects of γ-Tocotrienol on Invasion and Metastasis of Human Gastric Adenocarcinoma SGC-7901 Cells. J. Nutr. Biochem. 2010, 21, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.N.; Chang, P.N.; Han, H.Y.; Lee, D.T.W.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. γ-Tocotrienol Suppresses Prostate Cancer Cell Proliferation and Invasion through Multiple-Signalling Pathways. Br. J. Cancer 2008, 99, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, B.S.; Briski, K.P.; Tirmenstein, M.A.; Fariss, M.W.; Gapor, A.; Sylvester, P.W. Antiproliferative and Apoptotic Effects of Tocopherols and Tocotrienols on Normal Mouse Mammary Epithelial Cells. Lipids 2000, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of Nutrition on Neurodegenerative Diseases. A Systematic Review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Fattoretti, P.; Malavolta, M.; Fabbietti, P.; Papa, R.; Giacconi, R.; Costarelli, L.; Galeazzi, R.; Paoloni, C.; Postacchini, D.; Lattanzio, F.; et al. Oxidative Stress in Elderly with Different Cognitive Status: My Mind Project. J. Alzheimers Dis. 2018, 63, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.; Llewellyn, D.; Isaac, M.G.E.K.N.; Tabet, N. Vitamin E for Alzheimer’s Dementia and Mild Cognitive Impairment. Cochrane Database Syst. Rev. 2017, 2017, CD002854. [Google Scholar]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil. Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Hantke, B.; Lahmann, C.; Venzke, K.; Fischer, T.; Kocourek, A.; Jack Windsor, L.; Bergemann, J.; Stäb, F.; Tschesche, H. Influence of Flavonoids and Vitamins on the MMP- and TIMP-Expression of Human Dermal Fibroblasts after UVA Irradiation. Photochem. Photobiol. Sci. 2002, 1, 826–833. [Google Scholar] [CrossRef]
- Hasanov, H.; Mammadova, K.; Guliyeva, F.; Azizova, U.; Mikailova, N. The Role of Matrix Metalloproteinases in Human Body. Biol. Med. 2019, 11, 542. [Google Scholar]
- Ibuki, A.; Akase, T.; Nagase, T.; Minematsu, T.; Nakagami, G.; Horii, M.; Sagara, H.; Komeda, T.; Kobayashi, M.; Shimada, T.; et al. Skin Fragility in Obese Diabetic Mice: Possible Involvement of Elevated Oxidative Stress and Upregulation of Matrix Metalloproteinases. Exp. Dermatol. 2012, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Altuwaijri, S.; Yeh, S. RRR-α-Tocopheryl Succinate Inhibits Human Prostate Cancer Cell Invasiveness. Oncogene 2004, 23, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Adachi, Y.; Mitsushita, J.; Kuwabara, M.; Nagasawa, A.; Harada, S.; Furuta, S.; Zhang, Y.; Seheli, K.; Miyazaki, H.; et al. Reactive Oxygen Generated by NADPH Oxidase 1 (Nox1) Contributes to Cell Invasion by Regulating Matrix Metalloprotease-9 Production and Cell Migration. J. Biol. Chem. 2010, 285, 4481–4488. [Google Scholar] [CrossRef] [PubMed]
- Sanches, L.D.; Santos, S.A.A.; Carvalho, J.R.; Jeronimo, G.D.M.; Favaro, W.J.; Reis, M.D.G.; Felisbino, S.L.; Justulin, L.A. Protective Effect of γ-Tocopherol-Enriched Diet on N-Methyl-N-Nitrosourea-Induced Epithelial Dysplasia in Rat Ventral Prostate. Int. J. Exp. Pathol. 2013, 94, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Chu, S.C.; Yang, S.F.; Chen, P.N.; Liu, Y.C.; Lu, K.H. Silibinin Suppresses Human Osteosarcoma MG-63 Cell Invasion by Inhibiting the ERK-Dependent c-Jun/AP-1 Induction of MMP-2. Carcinogenesis 2007, 28, 977–987. [Google Scholar] [CrossRef]
- Weng, C.J.; Chau, C.F.; Hsieh, Y.S.; Yang, S.F.; Yen, G.C. Lucidenic Acid Inhibits PMA-Induced Invasion of Human Hepatoma Cells through Inactivating MAPK/ERK Signal Transduction Pathway and Reducing Binding Activities of NF-ΚB and AP-1. Carcinogenesis 2008, 29, 147–156. [Google Scholar] [CrossRef]
- Lin, M.L.; Lu, Y.C.; Chung, J.G.; Wang, S.G.; Lin, H.T.; Kang, S.E.; Tang, C.H.; Ko, J.L.; Chen, S.S. Down-Regulation of MMP-2 through the P38 MAPK-NF-ΚB-Dependent Pathway by Aloe-Emodin Leads to Inhibition of Nasopharyngeal Carcinoma Cell Invasion. Mol. Carcinog. 2010, 49, 783–797. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; Bhutani, J.; O’keefe, J.H. The Health Benefits of Vitamin K. Open Heart 2015, 2, e000300. [Google Scholar] [CrossRef]
- Maresz, K. Proper Calcium Use: Vitamin K2 as a Promoter of Bone and Cardiovascular Health. Integr. Med. 2015, 14, 34–39. [Google Scholar]
- Booth, S.L. Vitamin K: Food composition and dietary intakes. Food Nutr. Res. 2012, 56, 5505. [Google Scholar] [CrossRef]
- Holmes, M.V.; Hunt, B.J.; Shearer, M.J. The Role of Dietary Vitamin K in the Management of Oral Vitamin K Antagonists. Blood Rev. 2012, 26, 1–14. [Google Scholar] [CrossRef]
- de Souza, A.S.; Ribeiro, R.C.B.; Costa, D.C.S.; Pauli, F.P.; Pinho, D.R.; de Moraes, M.G.; da Silva, F.d.C.; Forezi, L.d.S.M.; Ferreira, V.F. Menadione: A platform and a target to valuable compounds synthesis. Beilstein J. Org. Chem. 2022, 18, 381–419. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Okano, T. Annual Review of Nutrition Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Thematic Review Series: Fat-Soluble Vitamins: Vitamin K: Recent Trends in the Metabolism and Cell Biology of Vitamin K with Special Reference to Vitamin K Cycling and MK-4 Biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef]
- Saupe, J.; Shearer, M.J.; Kohlmeier, M. Phylloquinone transport and its influence on gamma-carboxyglutamate residues of osteocalcin in patients on maintenance hemodialysis. Am. J. Clin. Nutr. 1993, 58, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Bach, A.; Kohlmeier, M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J. Nutr. 1996, 126, 1181S–1186S. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Differential Lipoprotein Transport Pathways of K-Vitamins in Healthy Subjects. Biochim. Biophys. Acta 2002, 1570, 27–32. [Google Scholar] [CrossRef]
- Thijssen, H.H.; Drittij-Reijnders, M.J. Vitamin K status in human tissues: Tissue-specific accumulation of phylloquinone and menaquinone-4. Br. J. Nutr. 1996, 75, 121–127. [Google Scholar] [CrossRef]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of Phylloquinone (Vitamin K1) into Menaquinone-4 (Vitamin K2) in Mice: Two Possible Routes for Menaquinone-4 Accumulation in Cerebra of Mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef]
- Toshiro, S.; Yutaka, O.; Yoko, Y.; Sanshiroh, S.; Hiroshi, H. Difference in the Metabolism of Vitamin K between Liver and Bone in Vitamin K-Deficient Rats. Br. J. Nutr. 2002, 87, 307–314. [Google Scholar]
- Shearer, M.J.; Mallinson, C.N.; Webster, G.R.; Barkhan, P. Clearance from plasma and excretion in urine, faeces and bile of an intravenous dose of tritiated vitamin K 1 in man. Br. J. Haematol. 1972, 22, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Barkhan, P. Studies on the metabolites of phylloquinone (vitamin K1) in the urine of man. Biochim. Biophys. Acta 1973, 297, 300–312. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Vitamin K, an Example of Triage Theory: Is Micronutrient Inadequacy Linked to Diseases of Aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Newman, P. Metabolism and Cell Biology of Vitamin K. Thromb. Haemost. 2008, 100, 530–547. [Google Scholar] [PubMed]
- Danziger, J. Vitamin K-Dependent Proteins, Warfarin, and Vascular Calcification. Clin. J. Am. Soc. Nephrol. 2008, 3, 1504–1510. [Google Scholar] [CrossRef]
- Tie, J.K.; Stafford, D.W. Functional Study of the Vitamin K Cycle Enzymes in Live Cells. Methods Enzymol. 2017, 584, 349–394. [Google Scholar]
- Berkner, K.L.; Runge, K.W. Vitamin K-Dependent Protein Activation: Normal Gamma-Glutamyl Carboxylation and Disruption in Disease. Int. J. Mol. Sci. 2022, 23, 5759. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, J.; Duan, L.; Li, S. Role of Emerging Vitamin K-Dependent Proteins: Growth Arrest-Specific Protein 6, Gla-Rich Protein and Periostin (Review). Int. J. Mol. Med. 2021, 47, 2. [Google Scholar] [CrossRef]
- Fusaro, M.; Mereu, M.C.; Aghi, A.; Iervasi, G.; Gallieni, M. Vitamin K and bone. Clin. Cases Miner. Bone Metab. 2017, 14, 200–206. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Anwar, F.; Khan, R.; Sachan, R.; Kazmi, I.; Rawat, A.; Sabih, A.; Singh, R.; Afzal, M.; Ahmad, A.; Al-Orab, A.S.; et al. Therapeutic Role of Calcium and Vitamin K3 in Chemically Induced Hepatocarcinogenesis–New Tools for Cancer Treatment. Arch. Physiol. Biochem. 2019, 125, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Iervasi, G.; la Manna, G.; et al. Vitamin K and Osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.S.; Bigman, G.; Rusu, M.E. The Role of Vitamin k in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Hiwatashi, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K Suppresses Lipopolysaccharide-Induced Inflammation in the Rat. Biosci. Biotechnol. Biochem. 2006, 70, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Booth, S.L.; Massaro, J.M.; Jacques, P.F.; D’Agostino, R.B.; Dawson-Hughes, B.; Ordovas, J.M.; O’Donnell, C.J.; Kathiresan, S.; Keaney, J.F.; et al. Vitamin K and Vitamin D Status: Associations with Inflammatory Markers in the Framingham Offspring Study. Am. J. Epidemiol. 2008, 167, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Itoh, S.; Morimoto, H. Stopped-flow kinetic study of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J. Biol. Chem. 1992, 267, 22277–22281. [Google Scholar] [CrossRef]
- Komai, M.; Shirakawa, H. Vitamin K metabolism. Menaquinone-4 (MK-4) formation from ingested VK analogues and its potent relation to bone function. Clin. Calcium. 2007, 17, 1663–1672. [Google Scholar]
- Card, D.J.; Gorska, R.; Cutler, J.; Harrington, D.J. Vitamin K Metabolism: Current Knowledge and Future Research. Mol. Nutr. Food. Res. 2014, 58, 1590–1600. [Google Scholar] [CrossRef]
- Akbari, S.; Rasouli-Ghahroudi, A.A. Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies. Biomed. Res. Int. 2018, 2018, 4629383. [Google Scholar] [CrossRef]
- Orlando, A.; Linsalata, M.; Tutino, V.; D’Attoma, B.; Notarnicola, M.; Russo, F. Vitamin K1 Exerts Antiproliferative Effects and Induces Apoptosis in Three Differently Graded Human Colon Cancer Cell Lines. Biomed. Res. Int. 2015, 2015, 296721. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Tuccinardi, D.; D’Onofrio, L.; Watanabe, M.; Maggi, D.; Maurizi, A.R.; Greto, V.; Buzzetti, R.; Napoli, N.; Pozzilli, P.; et al. Vitamin K and Osteoporosis: Myth or Reality? Metabolism 2017, 70, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K. Vitamins K1 and K2: The Emerging Group of Vitamins Required for Human Health. J. Nutr. Metab. 2017, 2017, 6254836. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C. Vitamin K Deficiency Bleeding: An Ounce of Prevention. Neonatal. Netw. 2020, 39, 356–362. [Google Scholar] [CrossRef]
- Kyla Shea, M.; Holden, R.M. Vitamin K Status and Vascular Calcification: Evidence from Observational and Clinical Studies. Adv. Nutr. 2012, 3, 158–165. [Google Scholar] [CrossRef]
- Shioi, A.; Morioka, T.; Shoji, T.; Emoto, M. The Inhibitory Roles of Vitamin K in Progression of Vascular Calcification. Nutrients 2020, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Saja, M.F.; Abdo, A.A.; Sanai, F.M.; Shaikh, S.A.; Gader, A.G.M.A. The Coagulopathy of Liver Disease: Does Vitamin K Help? Blood Coagul. Fibrinolysis 2013, 24, 10–17. [Google Scholar] [CrossRef]
- Jin, S.; Hong, L.; FakhriRavari, A. The Role of Vitamin K in Cirrhosis: Do Pharmaco-K-Netics Matter? Gastrointest. Disord. 2022, 4, 15–21. [Google Scholar] [CrossRef]
- Vermeer, C. Vitamin K: The Effect on Health beyond Coagulation—An Overview. Food Nutr. Res. 2012, 56, 5329. [Google Scholar] [CrossRef]
- Fenech, M. Vitamins Associated with Brain Aging, Mild Cognitive Impairment, and Alzheimer Disease: Biomarkers, Epidemiological and Experimental Evidence, Plausible Mechanisms, and Knowledge Gaps. Adv. Nutr. 2017, 8, 958–970. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Mett, J.; Hartmann, T. The Impact of Vitamin E and Other Fat-Soluble Vitamins on Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 1785. [Google Scholar] [CrossRef] [PubMed]
- Elshaikh, A.O.; Shah, L.; Mathew, C.J.; Lee, R.; Jose, M.T.; Cancarevic, I. Influence of Vitamin K on Bone Mineral Density and Osteoporosis. Cureus 2020, 12, e10816. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Olleros Rodríguez, C.; Díaz Curiel, M. Vitamin K and Bone Health: A Review on the Effects of Vitamin K Deficiency and Supplementation and the Effect of Non-Vitamin K Antagonist Oral Anticoagulants on Different Bone Parameters. J. Osteoporos. 2019, 2019, 269176. [Google Scholar] [CrossRef]
- Tang, S.; Ruan, Z.; Ma, A.; Wang, D.; Kou, J. Effect of Vitamin K on Wound Healing: A Systematic Review and Meta-Analysis Based on Preclinical Studies. Front. Pharmacol. 2022, 13, 1063349. [Google Scholar] [CrossRef] [PubMed]
- Chow, O.; Barbul, A. Immunonutrition: Role in Wound Healing and Tissue Regeneration. Adv. Wound Care 2014, 3, 46. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Vitamin K and the Immune System. In Nutrition and Immunity; Springer Cham: Scotland, UK, 2019; pp. 75–79. [Google Scholar]
- Di Monte, D.; Bellomo, G.; Thor, H.; Nicotera, P.; Orrenius, S. Menadione-Induced Cytotoxicity Is Associated with Protein Thiol Oxidation and Alteration in Intracellular Ca2+ Homeostasis. Arch. Biochem. Biophys. 1984, 235, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Y.H.; Chang, N.T.; Chen, W.J.; Juan, C.C. Vitamin K3-Induced Cell Cycle Arrest and Apoptotic Cell Death Are Accompanied by Altered Expression of c-Fos and c-Myc in Nasopharyngeal Carcinoma Cells. Oncogene 1993, 8, 2237–2244. [Google Scholar] [PubMed]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Powerful Micronutrient in Aging and Age-Related Diseases: Pros and Cons from Clinical Studies. Int. J. Mol. Sci. 2019, 20, 4150. [Google Scholar] [CrossRef] [PubMed]
- Kieronska-Rudek, A.; Kij, A.; Kaczara, P.; Tworzydlo, A.; Napiorkowski, M.; Sidoryk, K.; Chlopicki, S. Exogenous Vitamins k Exert Anti-Inflammatory Effects Dissociated from Their Role as Substrates for Synthesis of Endogenous Mk-4 in Murine Macrophages Cell Line. Cells 2021, 10, 1571. [Google Scholar] [CrossRef]
- Hariri, E.; Kassis, N.; Iskandar, J.P.; Schurgers, L.J.; Saad, A.; Abdelfattah, O.; Bansal, A.; Isogai, T.; Harb, S.C.; Kapadia, S. Vitamin K 2-a Neglected Player in Cardiovascular Health: A Narrative Review. Open Heart 2021, 8, e001715. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Maqbool, M.F.; Maryam, A.; Khan, M.; Shakir, H.A.; Irfan, M.; Ara, C.; Li, Y.; Ma, T. Vitamin K: A Novel Cancer Chemosensitizer. Biotechnol. Appl. Biochem. 2022, 69, 2641–2657. [Google Scholar] [CrossRef]
- Bouzahzah, B.; Nishikawa, Y.; Simon, D.; Carr, B.I. Growth Control and Gene Expression in a New Hepatocellular Carcinoma Cell Line, Hep40: Inhibitory Actions of Vitamin K. J. Cell. Physiol. 1995, 165, 459–467. [Google Scholar] [CrossRef]
- Mizuta, T.; Ozaki, I. Hepatocellular Carcinoma and Vitamin K. Vitam. Horm. 2008, 78, 435–442. [Google Scholar] [PubMed]
- Yu, D.W.; Li, Q.J.; Cheng, L.; Yang, P.F.; Sun, W.P.; Peng, Y.; Hu, J.J.; Wu, J.J.; Gong, J.P.; Zhong, G.C. Dietary Vitamin K Intake and the Risk of Pancreatic Cancer: A Prospective Study of 101,695 American Adults. Am. J. Epidemiol. 2021, 190, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Xv, F.; Chen, J.; Duan, L.; Li, S. Research Progress on the Anticancer Effects of Vitamin K2. Oncol. Lett. 2018, 15, 8926–8934. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and Molecular Mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Ozaki, I.; Zhang, H.; Mizuta, T.; Ide, Y.; Eguchi, Y.; Yasutake, T.; Sakamaki, T.; Pestell, R.G.; Yamamoto, K. Menatetrenone, a Vitamin K2 Analogue, Inhibits Hepatocellular Carcinoma Cell Growth by Suppressing Cyclin D1 Expression through Inhibition of Nuclear Factor ΚB Activation. Clin. Cancer Res. 2007, 13, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Osada, S.; Carr, B.I. Mechanism of Novel Vitamin K Analog Induced Growth Inhibition in Human Hepatoma Cell Line. J. Hepatol. 2001, 5, 676–682. [Google Scholar] [CrossRef]
- Osada, S.; Osada, K.; Carr, B.I. Tumor Cell Growth Inhibition and Extracellular Signal-Regulated Kinase (ERK) Phosphorylation by Novel K Vitamins. J. Mol. Biol. 2001, 314, 765–772. [Google Scholar] [CrossRef]
- Venugopal, M.; Jamison, J.M.; Gilloteaux, J.; Koch, J.A.; Summers, M.; Giammar, D.; Sowick, C.; Summers, J.L. Synergistic Antitumor Activity of Vitamins C and K3 on Human Urologic Tumor Cell Lines. Life Sci. 1996, 59, 1389–1400. [Google Scholar] [CrossRef]
- Chen, M.F.; Yang, C.M.; Su, C.M.; Liao, J.W.; Hu, M.L. Inhibitory Effect of Vitamin C in Combination with Vitamin K3 on Tumor Growth and Metastasis of Lewis Lung Carcinoma Xenografted in C57BL/6 Mice. Nutr. Cancer 2011, 63, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.; Elsebaey, S.; Allam, S.; El-Sisi, A. Modulating the Siah2-PHD3-HIF1α Axis and/or Autophagy Potentially Retard Colon Cancer Proliferation Possibly, Due to the Damping of Colon Cancer Stem Cells. Biomed. Pharmacother. 2022, 154, 113562. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Porras, A.R.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Vitamin K3 and Vitamin C Alone or in Combination Induced Apoptosis in Leukemia Cells by a Similar Oxidative Stress Signalling Mechanism. Cancer Cell Int. 2011, 11, 19. [Google Scholar] [CrossRef]
- Suresh, S.; Raghu, D.; Karunagaran, D. Menadione (Vitamin K3) Induces Apoptosis of Human Oral Cancer Cells and Reduces Their Metastatic Potential by Modulating the Expression of Epithelial to Mesenchymal Transition Markers and Inhibiting Migration. Asian Pac. J. Cancer Prev. 2013, 14, 5461–5465. [Google Scholar] [CrossRef] [PubMed]
- Degen, M.; Alexander, B.; Choudhury, M.; Eshghi, M.; Konno, S. Alternative Therapeutic Approach to Renal-Cell Carcinoma: Induction of Apoptosis with Combination of Vitamin K3 and D-Fraction. J. Endourol. 2013, 27, 1499–1503. [Google Scholar] [CrossRef]
- Vasefi, M.; Hudson, M.; Ghaboolian-Zare, E. Diet Associated with Inflammation and Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2019, 3, 299–309. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Troesch, B.; Weber, P. Inadequate Supply of Vitamins and DHA in the Elderly: Implications for Brain Aging and Alzheimer-Type Dementia. Nutrition 2015, 31, 261–275. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Noble, P.J.M.; Pagliocca, A.; Pritchard, D.M.; Hart, C.A.; Campbell, F.; Dodson, A.R.; Dockray, G.J.; Varro, A. Stimulation of MMP-7 (Matrilysin) by Helicobacter Pylori in Human Gastric Epithelial Cells: Role in Epithelial Cell Migration. J. Cell Sci. 2003, 116, 3017–3026. [Google Scholar] [CrossRef]
- Zhang, H.; Ozaki, I.; Hamajima, H.; Iwane, S.; Takahashi, H.; Kawaguchi, Y.; Eguchi, Y.; Yamamoto, K.; Mizuta, T. Vitamin K2 Augments 5-Fluorouracil-Induced Growth Inhibition of Human Hepatocellular Carcinoma Cells by Inhibiting NF-ΚB Activation. Oncol. Rep. 2010, 25, 159–166. [Google Scholar]








| Function | Activity | Related Disease | Ref. |
|---|---|---|---|
| Vision regulation | Rhodopsin generation | Night blindness | [86] |
| Cell growth and development | Regulation of gene expression | Infectious diseases | [87] |
| Immune regulation | Promotion of the growth and differentiation of cells for tissue repair Promotion of immune cell differentiation | Acute promyelocytic leukemia | [88,89,90] |
| Osteoporosis | [91,92] | ||
| Cellular communication | Inhibiting the production of proinflammatory cytokines Protection of immune cells from oxidative stress | Cancer | [17,18,93,94,95] |
| Obesity and insulin resistance | [96,97] | ||
| Reproduction | Assist the growth and maturation of follicles Regulation of the genes related to spermatogenesis | Congenital malformations | [98] |
| Diabetes mellitus and gestational diabetes | [99] | ||
| MMP regulation | Regulation of MMP-2 and MMP-9 expression | Cancers | [11,17,18,94,95,100,101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, H.V.T.; Nguyen, Y.T.; Kim, N.; Lee, H.J. Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity. Int. J. Mol. Sci. 2023, 24, 17038. https://doi.org/10.3390/ijms242317038
Vo HVT, Nguyen YT, Kim N, Lee HJ. Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity. International Journal of Molecular Sciences. 2023; 24(23):17038. https://doi.org/10.3390/ijms242317038
Chicago/Turabian StyleVo, Ha Vy Thi, Yen Thi Nguyen, Namdoo Kim, and Hyuck Jin Lee. 2023. "Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity" International Journal of Molecular Sciences 24, no. 23: 17038. https://doi.org/10.3390/ijms242317038