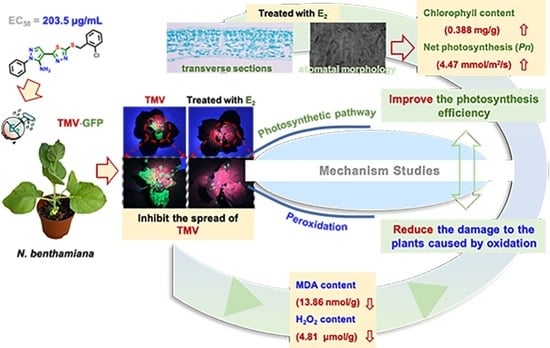
Novel 1,3,4-Thiadiazole Derivatives: Synthesis, Antiviral Bioassay and Regulation the Photosynthetic Pathway of Tobacco against TMV Infection
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Antiviral Activity
2.3. Effect of E2 against TMV Spread in N. benthamiana
2.4. Tissue Morphology Observed in Paraffin Sections
2.5. Photosynthesis and Gas Exchange
2.6. Stomatal Analysis of Tobacco Leaves
2.7. Chlorophyll, H2O2 and MDA Content Analysis in Tobacco Leaves
3. Discussion
4. Materials and Methods
4.1. Instruments and Materials
4.2. Plants, TMV, and TMV-GFP
4.3. Synthetic Procedure [39,40,41]
4.3.1. General Procedure for the Preparation of Intermediate B
4.3.2. General Procedure for the Preparation of Intermediate C
4.3.3. General Procedure for the Preparation of Intermediate D
4.3.4. General Procedure for the Preparation of Title Compounds E1–E28
4.4. In Vivo Antiviral Bioassay against TMV
4.5. Verification of the Protective Activity of E2 on N. benthamiana with TMV-GFP
4.6. N. benthamiana with Different Treatments for a Mechanism Study
4.7. Plant Tissue Morphological Observation
4.8. Physiological Parameter Measurement and Stomatal Observation for the Host
4.9. Determination of Chlorophyll, H2O2 and Malondialdehyde (MDA) Contents
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, Y.H.; Liu, K.X.; Zhang, J.X.; Mu, S.Z.; Hao, X.J. The limonoids and their anti-tobacco mosaic virus (TMV) activities from munronia unifoliolata oliv. J. Agric. Food Chem. 2012, 60, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Lv, X.; He, L.; Shi, H.; Liao, S.; Liu, C.; Huang, Q.; Li, X.; He, X.; Chen, H.; et al. Dual-Action Pesticide Carrier That Continuously Induces Plant Resistance, Enhances Plant Anti-Tobacco Mosaic Virus Activity, and Promotes Plant Growth. J. Agric. Food Chem. 2019, 67, 10000–10009. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Yu, M.; Meng, L.; Zhou, T.; Shan, Y.; Liu, X.; Xia, Z.; An, M.; Wu, Y. Transcriptomic and Functional Analyses Indicate Novel Anti-viral Mode of Actions on Tobacco Mosaic Virus of a Microbial Natural Product ε-Poly-l-lysine. J. Agric. Food Chem. 2021, 69, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.Y.; Yu, M.; Wang, K.H.; Zhu, B.B.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Wang, Q.M. Discovery of glyantrypine-family alkaloids as novel antiviral and anti-phytopathogenic-fungus agents. Pest Manag. Sci. 2022, 78, 982–990. [Google Scholar] [CrossRef]
- Bwalya, J.; Alazem, M.; Kim, K. Photosynthesis-related genes induce resistance against soybean mosaic virus: Evidence for involvement of the RNA silencing pathway. Mol. Plant Pathol. 2022, 23, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Spetz, C.; Li, L.; Wang, X. Comparative transcriptome analysis in Triticum aestivum infecting wheat dwarf virus reveals the effects of viral infection on phytohormone and photosynthesis metabolism pathways. Phytopathol. Res. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Yang, H.; Luo, P. Changes in Photosynthesis Could Provide Important Insight into the Interaction between Wheat and Fungal Pathogens. Int. J. Mol. Sci. 2021, 22, 8865. [Google Scholar] [CrossRef]
- Matsumura, H.; Shiomi, K.; Yamamoto, A.; Taketani, Y.; Kobayashi, N.; Yoshizawa, T.; Tanaka, S.-I.; Yoshikawa, H.; Endo, M.; Fukayama, H. Hybrid Rubisco with Complete Replacement of Rice Rubisco Small Subunits by Sorghum Counterparts Confers C4 Plant-like High Catalytic Activity. Mol. Plant 2020, 13, 1570–1581. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, H.; Song, J.; Yang, Y.; Hu, X.; Wiyao, K.T.; Zhai, Z. Applying spectral fractal dimension index to predict the SPAD value of rice leaves under bacterial blight disease stress. Plant Methods 2022, 18, 67. [Google Scholar] [CrossRef]
- Tobar, M.; Fiore, N.; Pérez-Donoso, A.G.; León, R.; Rosales, I.M.; Gambardella, M. Divergent molecular and growth responses of young “Cabernet Sauvignon” (Vitis vinifera) plants to simple and mixed infections with Grapevine rupestris stem pitting-associated virus. Hortic. Res. 2020, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Huseynova, I.M.; Mirzayeva, S.M.; Sultanova, N.F.; Aliyeva, D.R.; Mustafayev, N.S.; Aliyev, J.A. Virus-induced changes in photosynthetic parameters and peroxidase isoenzyme contents in tomato (Solanum lycopersicum L.) plants. Photosynthetica 2018, 56, 841–850. [Google Scholar] [CrossRef]
- Zine, H.; Rifai, L.A.; Koussa, T.; Bentiss, F.; Guesmi, S.; Laachir, A.; Makroum, K.; Belfaiza, M.; Faize, M. The mononuclear nickel (II) complex bis(azido-κN) bis [2,5-bis(pyridin-2-yl)-1,3,4-thiadiazole-κ2N2, N3] nickel (II) protects tomato from verticil-lium dahliae by inhibiting fungal growth and activating plant defences. Pest Manag. Sci. 2017, 73, 188–197. [Google Scholar] [CrossRef]
- Bai, S.; Zhu, Y.; Wu, Q. Asymmetric Mannich Reaction: Synthesis of Novel Chiral 5-(substituted aryl)-1,3,4-Thiadiazole Derivatives with Anti-Plant-Virus Potency. Heterocycl. Commun. 2019, 25, 47–51. [Google Scholar] [CrossRef]
- Yang, R.; Han, M.; Fan, J.; Cheng, W.; Ma, N.; Yan, X.; Guo, Y. Development of Novel (+)-Nootkatone Thioethers Containing 1,3,4-Oxadiazole/Thiadiazole Moieties as Insecticide Candidates against Three Species of Insect Pests. J. Agric. Food Chem. 2021, 69, 15544–15553. [Google Scholar] [CrossRef]
- Zoroddu, S.; Corona, P.; Sanna, L.; Borghi, F.; Bordoni, V.; Asproni, B.; Pinna, G.A.; Bagella, L.; Murineddu, G. Novel 1,3,4-oxadiazole chalcogen analogues: Synthesis and cytotoxic activity. Eur. J. Med. Chem. 2022, 238, 114440. [Google Scholar] [CrossRef]
- Zhang, P.L.; Gopala, L.; Zhang, S.L.; Cai, G.X.; Zhou, C.H. An unanticipated discovery towards novel naphthalimide cor-belled aminothiazoximes as potential anti-MRSA agents and allosteric modulators for PBP2a. Eur. J. Med. Chem. 2022, 229, 114050. [Google Scholar] [CrossRef]
- Yu, L.; Gan, X.; Zhou, D.; He, F.; Zeng, S.; Hu, D. Synthesis and Antiviral Activity of Novel 1,4-Pentadien-3-one Derivatives Containing a 1,3,4-Thiadiazole Moiety. Molecules 2017, 22, 658. [Google Scholar] [CrossRef]
- Green, D.S.; Kruger, E.L. Light-mediated constraints on leaf function correlate with leaf structure among deciduous and evergreen tree species. Tree Physiol. 2001, 21, 1341–1346. [Google Scholar] [CrossRef]
- Yu, M.; Chen, L.; Liu, D.-H.; Sun, D.; Shi, G.-L.; Yin, Y.; Wen, D.-Q.; Wang, Z.-X.; Ai, J. Enhancement of Photosynthetic Capacity in Spongy Mesophyll Cells in White Leaves of Actinidia kolomikta. Front. Plant Sci. 2022, 13, 856732. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.-G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Borsuk, A.M.; Brodersen, C.R. The Spatial Distribution of Chlorophyll in Leaves. Plant Physiol. 2019, 180, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Koiwa, H.; Kojima, K.; Ikeda, T.; Yoshida, Y. Fluctuations of particles on chioroplast thylakoid membranes in tomato plants infected with virulent or attenuated strain of tobacco mosaic virus. Ann. Phytopath. Soc. Japan 1992, 58, 58–64. [Google Scholar] [CrossRef]
- Sayed, O. Chlorophyll Fluorescence as a Tool in Cereal Crop Research. Photosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Guo, Y.P.; Guo, D.P.; Peng, Y.; Chen, J.S. Photosynthetic responses of radish (Raphanus sativus var. longipinnatus) plants to infection by turnip mosaic virus. Photosynthetica 2005, 43, 457–462. [Google Scholar] [CrossRef]
- Guo, D.-P.; Guo, Y.-P.; Zhao, J.-P.; Liu, H.; Peng, Y.; Wang, Q.-M.; Chen, J.-S.; Rao, G.-Z. Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus infection. Plant Sci. 2005, 168, 57–63. [Google Scholar] [CrossRef]
- Ryšlavá, H.; Müller, K.; Semorádová, Š.; Synková, H.; Čeřovská, N. Photosynthesis and Activity of Phosphoenolpyruvate carboxylase in Nicotiana tabacum L. Leaves Infected by Potato virus A and Potato virus Y. Photosynthetica 2003, 41, 357–363. [Google Scholar] [CrossRef]
- Sampol, B.; Bota, J.; Riera, D.; Medrano, H.; Flexas, J. Analysis of the virus-induced inhibition of photosynthesis in malmsey grapevines. New Phytol. 2003, 160, 403–412. [Google Scholar] [CrossRef]
- Kalaichelvi, K.; Prabhaharan, J.; Rao, D.S. Physiology of Bhendi Yellow Vein Mosaic Disease Infected Plant. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2284–2288. [Google Scholar] [CrossRef]
- Murray, R.R.; Emblow, M.S.M.; Hetherington, A.M.; Foster, G.D. Plant virus infections control stomatal development. Sci. Rep. 2016, 6, srep34507. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2013, 65, 1241–1257. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous Proline and Glycine Betaine Mediated Upregulation of Antioxidant Defense and Glyoxalase Systems Provides Better Protection against Salt-Induced Oxidative Stress in Two Rice (Oryza sativa L.) Varieties. BioMed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef] [PubMed]
- Song, X.S.; Wanga, Y.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Nogue, S.; Yu, J.Q. Effects of cucumber mosaic virus infection on electrontransport and antioxidant system in chloroplasts andmitochondria of cucumber and tomato leaves. Physiol. Plant 2009, 135, 246–257. [Google Scholar] [CrossRef]
- Madhusudhan, K.; Srikanta, B.; Shylaja, M.; Prakash, H.; Shetty, H. Changes in antioxidant enzymes, hydrogen peroxide, salicylic acid and oxidative stress in compatible and incompatible host-tobamovirus interaction. J. Plant Interactions 2009, 4, 157–166. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxifi-cation system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588. [Google Scholar] [CrossRef]
- Flefel, E.M.; Tantawy, W.A.; Abdel-Mageid, R.E.; Amr, A.E.-G.E.; Nadeem, R. Synthesis and anti-viral activities of some 3-(naphthalen-1-ylmethylene)-5-phenylfuran-2(3H)-one candidates. Res. Chem. Intermed. 2014, 40, 1365–1381. [Google Scholar] [CrossRef]
- Reddy, S.R.; Reddy, C.V.R.; Satyanarayana, B. Synthesis and Characterization of Novel Pyridine Associated 1,2,4-Triazolo-1,3,4-thiadiazines. Asian J. Chem. 2016, 28, 1708–1712. [Google Scholar] [CrossRef]
- Karabanovich, G.; Zemanova, J.; Smutny, T.; Szekely, R.; Sarkan, M.; Centarova, I.; Vocat, A.; Pavkova, I.; Conka, P.; Nemecek, J.; et al. Development of 3,5-dinitrobenzylsulfanyl-1,3,4-oxadiazoles and thiadiazoles as selective antitubercular agents active against replicating and nonreplicating mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 2362–2380. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.H.; Chen, J.; Di, Y.T.; Fang, X.; Dong, J.H.; Sang, P.; Wang, Y.H.; He, H.P.; Zhang, Z.K.; Hao, X.J. Anti-tobacco mosaic virus (TMV) quassinoids from Brucea javanica (L.) merr. J. Agric. Food Chem. 2010, 58, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.Y.; Xie, B.T.; Qin, Z.; Li, A.X.; Dong, S.X.; Zhang, H.H.; Duan, W.X.; Zhang, L.M.; Wang, Q.M. Sweet potato leaf curl virus decreased storage root yield and quality of edible sweetpotato in China. Agron. J. 2020, 112, 3948–3962. [Google Scholar] [CrossRef]










| NO. |  | Inhibition Rate ± SD (%) | ||
|---|---|---|---|---|
| R | Curative Activity | Protective Activity | Inaction Activity | |
| E1 | 3-CH3-benzyl | 18.0 ± 3.9 | 35.7 ± 4.4 | 25.0 ± 6.4 |
| E2 | 2-Cl-benzyl | 50.6 ± 2.2 | 65.1 ± 4.1 | 55.0 ± 1.6 |
| E3 | 4-Cl-benzyl | 35.6 ± 4.2 | 30.5 ± 4.4 | 48.0 ± 9.1 |
| E4 | 3-Cl-benzyl | 38.0 ± 8.5 | 39.6 ± 9.0 | 19.5 ± 8.6 |
| E5 | 2-CH3-benzyl | 41.7 ± 5.5 | 57.5 ± 9.6 | 0 |
| E6 | 4-CH3-benzyl | 44.6 ± 5.0 | 37.4 ± 7.0 | 32.1 ± 6.1 |
| E7 | 2-F-benzyl | 46.8 ± 4.8 | 30.9 ± 5.7 | 0 |
| E8 | 3-OCH3-benzyl | 59.2 ± 6.6 | 43.5 ± 1.1 | 23.7 ± 6.1 |
| E9 | 4-OCH3-benzyl | 50.5 ± 8.4 | 50.7 ± 8.4 | 42.5 ± 9.8 |
| E10 | 2-CF3-benzyl | 35.6 ± 4.2 | 57.7 ± 9.4 | 0 |
| E11 | 3-CF3-benzyl | 49.1 ± 7.9 | 45.0 ± 5.7 | 40.5 ± 5.9 |
| E12 | 4-CF3-benzyl | 0 | 48.2 ± 6.0 | 62.0 ±1.6 |
| E13 | CH-CH=CH2 | 29.3 ± 5.0 | 49.6 ± 8.5 | 32.0 ± 15.3 |
| E14 | Ethyl | 33.0 ± 9.6 | 63.0 ± 8.0 | 24.0 ± 5.3 |
| E15 | Benzyl | 0 | 43.2 ± 5.4 | 0 |
| E16 | 4-F-benzyl | 32.1 ± 4.7 | 46.4 ± 7.0 | 25.5 ± 9.4 |
| E17 | 2-OCH3-benzyl | 0 | 61.3 ± 8.7 | 0 |
| E18 | 3-F-benzyl | 0 | 29.0 ± 4.9 | 0 |
| E19 | 2-Br-benzyl | 0 | 45.9 ± 7.8 | 44.2 ± 12.9 |
| E20 | 3-Br-benzyl | 52.2 ± 6.6 | 42.4 ± 6.1 | 0 |
| E21 | 4-Br-benzyl | 50.5 ± 5.6 | 56.6 ± 4.1 | 34.5 ± 13.5 |
| E22 | i-Bu | 27.0 ± 5.4 | 47.5 ± 3.6 | 0 |
| E23 | n-Pr | 37.7 ± 6.5 | 0 | 13.0 ± 10.0 |
| E24 | 4-NO2-benzyl | 33.1 ± 6.0 | 41.7 ± 6.1 | 0 |
| E25 | 2-NO2-benzyl | 36.2 ± 6.4 | 35.3 ± 5.9 | 67.1 ± 7.2 |
| E26 | 3-NO2-benzyl | 0 | 48.7 ± 2.8 | 0 |
| E27 | Methyl | 32.1 ± 8.5 | 41.6 ± 2.4 | 23.4 ± 7.4 |
| E28 | n-Pentyl | 0 | 47.3 ± 3.5 | 52.3 ± 8.3 |
| NNM * | —— | 58.0 ± 6.6 | 54.6 ± 4.0 | 89.1 ± 6.8 |
| NO. | EC50 | Regression Equation | R2 |
|---|---|---|---|
| E2 | 393.3 ± 6.2 | y = 0.531x + 3.622 | 0.9747 |
| E8 | 285.7 ± 6.2 | y = 0.6374x + 3.4346 | 0.9311 |
| E9 | 449.3 ± 7.4 | y = 0.5284x + 3.5984 | 0.9746 |
| E20 | 400.6 ± 7.8 | y = 0.4949x + 3.7119 | 0.9928 |
| E21 | 462.1 ± 5.4 | y = 0.4188x + 3.884 | 0.9970 |
| NNM * | 158.0 ± 6.7 | y = 0.5066x + 3.8861 | 0.9552 |
| NO. | EC50 | Regression Equation | R2 |
|---|---|---|---|
| E2 | 203.5 ± 5.1 | y = 0.6718x + 3.4491 | 0.9094 |
| E10 | 321.2 ± 5.4 | y = 0.6182x + 3.4503 | 0.9590 |
| E14 | 240.8 ± 7.3 | y = 0.7418x + 3.2332 | 0.9550 |
| E17 | 259.7 ± 6.7 | y = 0.5995x + 3.5525 | 0.9025 |
| E21 | 312.7 ± 3.9 | y = 0.6182x + 3.4571 | 0.9871 |
| NNM * | 261.4 ± 4.5 | y = 0.4536x + 3.9035 | 0.9917 |
| Stomata Parameters | CK Group | T Group | S Group | TS Group |
|---|---|---|---|---|
| Stomata area (μm2) a | 0.82 ± 0.18 b | 1.17 ± 0.14 | 0.98 ± 0.26 | 1.02 ± 0.06 |
| Stomata aperture (μm) a | 0.26 ± 0.02 | 0.33 ± 0.03 | 0.35 ± 0.07 | 0.27 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Wen, F.; Zhang, C.; Luo, R.; Wu, Z. Novel 1,3,4-Thiadiazole Derivatives: Synthesis, Antiviral Bioassay and Regulation the Photosynthetic Pathway of Tobacco against TMV Infection. Int. J. Mol. Sci. 2023, 24, 8881. https://doi.org/10.3390/ijms24108881
Zheng H, Wen F, Zhang C, Luo R, Wu Z. Novel 1,3,4-Thiadiazole Derivatives: Synthesis, Antiviral Bioassay and Regulation the Photosynthetic Pathway of Tobacco against TMV Infection. International Journal of Molecular Sciences. 2023; 24(10):8881. https://doi.org/10.3390/ijms24108881
Chicago/Turabian StyleZheng, Huanlin, Fanglin Wen, Chengzhi Zhang, Rui Luo, and Zhibing Wu. 2023. "Novel 1,3,4-Thiadiazole Derivatives: Synthesis, Antiviral Bioassay and Regulation the Photosynthetic Pathway of Tobacco against TMV Infection" International Journal of Molecular Sciences 24, no. 10: 8881. https://doi.org/10.3390/ijms24108881