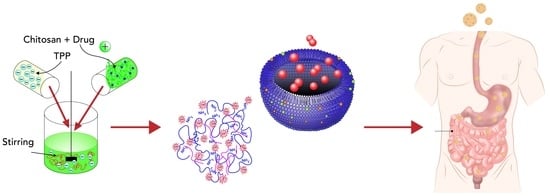
Chitosan Nanoparticles as Oral Drug Carriers
Abstract
:1. Introduction
2. Drug Administration
Drug Delivery Systems
3. Non-Oral Administration Routes
4. Oral Administration
4.1. Gastrointestinal Tract Structure
4.2. Devices and Materials
5. Ingestion of Nanoparticles
6. Polymeric Nanoparticles
6.1. Preparation of Polymeric Nanoparticles
6.1.1. Ionic Cross-Link Method
6.1.2. Covalent Cross-Link Method
6.1.3. Reverse Micellar Method
6.1.4. Precipitation/Coacervation
6.1.5. Emulsion–Droplet Coalescence Method
7. Chitosan Nanoparticles
7.1. Applications of Chitosan Nanoparticles for Oral Drug Delivery
7.1.1. Gene Delivery
7.1.2. Protein and Peptide Delivery
7.1.3. Drugs
7.1.4. Vaccines
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathias, N.R.; Hussain, M.A. Non-invasive systemic drug delivery: Developability considerations for alternate routes of administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cheng, Y.; Li, M.; Gref, R.; Chen, X. Smart Polymeric Nanocarriers. J. Nanomater. 2016, 2016, 3710921. [Google Scholar] [CrossRef]
- Ramadhani, N.; Shabir, M.; McConville, C. Preparation and characterisation of Kolliphor®P 188 and P 237 solid dispersion oral tablets containing the poorly water soluble drug disulfiram. Int. J. Pharm. 2014, 475, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Kasiramar, G. An Overview on Polymeric Nanoparticles used in the treatment of Diabetes Mellitus. Pharmatutor 2019, 5, 40. [Google Scholar]
- Ghadi, A.; Mahjoub, S.; Tabandeh, F.; Talebnia, F. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. Casp. J. Intern. Med. 2014, 5, 156–161. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Wang, J.J.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.L. Pharmacologic Principles. In Equine Internal Medicine, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 79–137. [Google Scholar]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Seidling, H.M.; Lampert, A.; Lohmann, K.; Schiele, J.T.; Send, A.J.F.; Witticke, D.; Haefeli, W.E. Safeguarding the process of drug administration with an emphasis on electronic support tools. Br. J. Clin. Pharmacol. 2013, 76, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Peña-Juárez, M.C.; Guadarrama-Escobar, O.R.; Escobar-Chávez, J.J. Transdermal Delivery Systems for Biomolecules. J. Pharm. Innov. 2021, 17, 319–332. [Google Scholar] [CrossRef]
- Singla, V.; Saini, S.; Joshi, B.; Rana, A.C. Emulgel: A New Platform for Topical Drug Delivery. Int. J. Pharma Bio Sci. 2012, 3, 485–498. [Google Scholar]
- Berlin, J.; May-McCarver, D.G.; Notterman, D.A.; Ward, R.M.; Weismann, D.N.; Wilson, G.S.; Wilson, J.T.; Bennett, D.R.; Hoskins, I.A.; Kaufman, P.; et al. Alternative Routes of Drug Administration-Advantages and Disadvantages (Subject Review). Pediatrics 1997, 100, 143–152. [Google Scholar]
- Subcutaneous Drug Administration. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 1994; pp. 35–45.
- Kumari, B. Ocular drug delivery system: Approaches to improve ocular bioavailability. GSC Biol. Pharm. Sci. 2019, 6, 001–010. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Coppola, A.; Cavalli, F.; Ora, J.; Puxeddu, E.; Matera, M.G.; Cazzola, M. Optimizing drug delivery in COPD: The role of inhaler devices. Respir. Med. 2017, 124, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Yurdasiper, A.; Arici, M.; Ozyazici, M. Nanopharmaceuticals. In Emerging Nanotechnologies in Immunology: The Design, Applications and Toxicology of Nanopharmaceuticals and Nanovaccines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 165–201. [Google Scholar]
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery—A review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef]
- Dupeyrón, D.; Rieumont, J.; González, M.; Castaño, V.M. Protein Delivery by Enteric Copolymer Nanoparticles. J. Disper. Sci. Technol. 2009, 30, 1188–1194. [Google Scholar] [CrossRef]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardakjian, B.L. Gastrointestinal system. Biomed. Imaging 2003, 2, 6-1–6-13. [Google Scholar]
- Martínez Bueno, C. Physiology of intestinal absorption. Rev. Esp. Enferm. Apar. Dig. 1966, 25, 933–941. [Google Scholar] [PubMed]
- Zia, L.; Johnson, I.; Mansurov, B.; Morgan, J.; Redi, M.; Saez-Trumper, D.; Taraborelli, D. Knowledge Gaps—Wikimedia Research 2030. Int. J. Biomed. Nanosci. Nanotechnol. 2019, 1–8. [Google Scholar]
- Salama, N.N.; Eddington, N.D.; Fasano, A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 15–28. [Google Scholar] [CrossRef]
- Johnson, L.R. Physiology of the Gastrointestinal Tract, 4th ed.; Barrett, K., Johnson, L., Ghishan, F., Merchant, J., Said, H., Wood, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Available online: https://www.elsevier.com/books/physiology-of-the-gastrointestinal-tract/johnson/978-0-12-088394-3 (accessed on 4 November 2022).
- Magalhães, J.; Vieira, A.; Santos, S.; Pinheiro, M.; Reis, S. Oral Administration of Nanoparticles-Based TB Drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 307–326. [Google Scholar]
- Omidian, H.; Park, K. Swelling agents and devices in oral drug delivery. J. Drug Deliv. Sci. Technol. 2008, 18, 83–93. [Google Scholar] [CrossRef]
- Jain, V.; Gupta, A.; Pawar, V.K.; Asthana, S.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Chitosan-Assisted Immunotherapy for Intervention of Experimental Leishmaniasis via Amphotericin B-Loaded Solid Lipid Nanoparticles. Biotechnol. Appl. Biochem. 2014, 174, 1309–1330. [Google Scholar] [CrossRef] [PubMed]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric plant-derived excipients in drug delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.P.; Alonso, M.J.; Csaba, N. Polymer-based oral peptide nanomedicines. Ther. Deliv. 2012, 3, 657–668. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Xie, H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Env. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Atyabi, F.; Dinarvand, R. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study. Int. J. Nanomed. 2012, 7, 1851–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, C.E.; Torr, E.E.; Mohd Jamili, N.H.; Bosquillon, C.; Sayers, I. Evaluation of Differentiated Human Bronchial Epithelial Cell Culture Systems for Asthma Research. J. Allergy Clin. Immunol. 2019, 6, 975–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, T.J.; Baker, M.A.B. Nanotechnology: Building and Observing at the Nanometer Scale. In Pharmacognosy: Fundamentals, Applications and Strategy; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 633–643. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Napier, M.E.; DeSimone, J.M. Nanoparticle drug delivery platform. Polym. Rev. 2007, 47, 321–327. [Google Scholar] [CrossRef]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nan.noparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Katas, H.; Raja, M.A.G.; Lam, K.L. Development of Chitosan Nanoparticles as a Stable Drug Delivery System for Protein/siRNA. Int. J. Biomater. 2013, 2013, 146320. [Google Scholar] [CrossRef] [Green Version]
- Fide, S.; Karaismailoglu, S.; Derman, S. Synthesis and characterization methods of polymeric nanoparticles 2. Preparation Methods of Polymeric Nanoparticles. Eur. J. Pharm. Sci. 2018, 1, 1–9. [Google Scholar]
- Campos, E.; Oliveira, J.L.; Da Silva, C.M.G.; Pascoli, M.; Pasquoto, T.; Lima, R.; Abhilash, P.C.; Fraceto, L.F.U. Polymeric and Solid Lipid Nanoparticles for Sustained Release of Carbendazim and Tebuconazole in Agricultural Applications. Sci. Rep. 2015, 5, srep13809. [Google Scholar] [CrossRef] [Green Version]
- Son, G.H.; Lee, B.J.; Cho, C.W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.V. Biopolymers in Drug Delivery: A Review. Pharmacologyonline 2011, 674, 666–674. [Google Scholar]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 58, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles Potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum. Vaccines Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Shah, K.W.; Xu, J. Synthesis, morphologies and building applications of nanostructured polymers. Polymers 2017, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Allémann, E.; Gurny, R.; Doelker, E. Drug-loaded nanoparticles—Preparation methods and drug targeting issues. Eur. J. Pharm. Biopharm. 1993, 39, 173–191. [Google Scholar]
- Kumari, R.M.; Sharma, N.; Gupta, N.; Chandra, R.; Nimesh, S. Synthesis and evolution of polymeric nanoparticles. In Design and Development of New Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–438. [Google Scholar]
- Pineda-Álvarez, R.A.; Bernad-Bernad, M.J.; Rodríguez-Cruz, I.M.; Escobar-Chávez, J.J. Development and Characterization of Starch/Gelatin Microneedle Arrays Loaded with Lecithin–Gelatin Nanoparticles of Losartan for Transdermal Delivery. J. Pharm. Innov. 2020, 17, 71–84. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. Available online: https://www.sciencedirect.com/science/article/pii/B9780081019757000051 (accessed on 14 October 2022).
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C.; Alcock, E.; Buttimer, F.; Schmidt, M.; Clarke, D.; Pemble, M.; Bardosova, M. Synthesis and characterisation of cross-linked chitosan composites functionalised with silver and gold nanoparticles for antimicrobial applications. Sci. Technol. Adv. Mater. 2017, 18, 528–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, P.; Tran, T.T.-D.; Zhang, J.; Kong, L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallury, P.; Kar, S.; Bamrungsap, S.; Huang, Y.-F.; Tan, W.; Santra, S. Ultra-small water-dispersible fluorescent chitosan nanoparticles: Synthesis, characterization and specific targeting. Chem. Commun. 2009, 17, 2347–2349. [Google Scholar] [CrossRef] [PubMed]
- Lorke, S.; Müller, U.; Meissl, R.; Brüggemann, O. Covalent cross-linking of polymers at room temperature. Int. J. Adhes. Adhes. 2019, 91, 150–159. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Saikia, C.; Gogoi, P. Chitosan: A Promising Biopolymer in Drug Delivery Applications. Mol. Genet. Genomic. Med. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Ahmed, A.B.; Konwar, R.; Sengupta, R. Atorvastatin calcium loaded chitosan nanoparticles: In vitro evaluation and in vivo pharmacokinetic studies in rabbits. Braz. J. Pharm. 2015, 51, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.P.; Mahapatra, S.K.; Sahu, S.K.; Pramanik, P.; Roy, S. Antioxidative effect of folate-modified chitosan nanoparticles. Asian Pac. J. Trop. Biomed. 2011, 1, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric Nanoparticle Technologies for Oral Drug Delivery. Clin. Gastroenterol. Hepatol. 2014, 12, 1605–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peniche, H.; Peniche, C. Chitosan nanoparticles: A contribution to nanomedicine. Polym. Int. 2011, 60, 883–889. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Bikiaris, D.; Avgoustakis, K.; Karavas, E.; Georgarakis, M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr. Polym. 2008, 73, 44–54. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef]
- Rosch, J.G.; Winter, H.; DuRoss, A.N.; Sahay, G.; Sun, C. Inverse-Micelle Synthesis of Doxorubicin-Loaded Alginate/Chitosan Nanoparticles and In Vitro Assessment of Breast Cancer Cytotoxicity. J. Colloid Interface Sci. 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [Green Version]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Saeed, R.M.; Dmour, I.; Taha, M.O. Stable Chitosan-Based Nanoparticles Using Polyphosphoric Acid or Hexametaphosphate for Tandem Ionotropic/Covalent Crosslinking and Subsequent Investigation as Novel Vehicles for Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J. Drug Target 2018, 26, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhang, L.; Zheng, J.; Kawashima, Y. A study of insulin-chitosan complex nanoparticles used for oral administration. Drug Deliv. Sci. Technol. 2004, 14, 435–439. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Kenechukwu, F.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Li, M.; Zhang, L. A cell-penetrating peptide mediated chitosan nanocarriers for improving intestinal insulin delivery. Carbohydr. Polym. 2017, 174, 182–189. [Google Scholar] [CrossRef]
- Lazaridou, M.; Christodoulou, E.; Nerantzaki, M.; Kostoglou, M.; Lambropoulou, D.A.; Katsarou, A.; Pantopoulos, K.; Bikiaris, D.N. Formulation and in-vitro characterization of chitosan-nanoparticles loaded with the iron chelator deferoxamine mesylate (DFO). Pharmaceutics 2020, 12, 238. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; He, J. Simultaneous release of hydrophilic and hydrophobic drugs from modified chitosan nanoparticles. Mater. Lett. 2015, 161, 415–418. [Google Scholar] [CrossRef]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125. [Google Scholar] [CrossRef]
- Shailender, J.; Ravi, P.R.; Sirukuri, M.R.; Dalvi, A.; Priya, O.K. Chitosan nanoparticles for the oral delivery of tenofovir disoproxil fumarate: Formulation optimization, characterization and ex vivo and in vivo evaluation for uptake mechanism in rats. Drug Dev. Ind. Pharm. 2018, 44, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, X.; Liu, C.; Zhang, X.; Zhang, X.; Chen, X.; Kou, Y.; Mao, S. Chitosan based polymer-lipid hybrid nanoparticles for oral delivery of enoxaparin. Int. J. Pharm. 2018, 547, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guan, Z.-Y.; Zhu, W.-F.; Zhong, L.-Y.; Qiu, Z.-Q.; Yue, P.-F.; Wu, W.-T.; Liu, J.; Huang, X. Preparation of puerarin chitosan oral nanoparticles by ionic gelation method and its related kinetics. Pharmaceutics 2020, 12, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.K.; Parikh, R.H.; Aboti, P.S. Development of Oral Sustained Release Rifampicin Loaded Chitosan Nanoparticles by Design of Experiment. J. Drug Deliv. 2013, 2013, 370938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Na Bhuket, P.R.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, F.; Tamjid, E.; Khajeh, K. Development of chitosan coated calcium-alginate nanocapsules for oral delivery of liraglutide to diabetic patients. Int. J. Biol. Macromol. 2018, 120, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Shi, X.-W.; Yang, G.-F.; Gong, L.-L.; Yuan, H.-Y.; Cui, Y.-J.; Wang, Y.; Du, Y.-M.; Li, Y. Chitosan nanoparticle as gene therapy vector via gastrointestinal mucosa administration: Results of an in vitro and in vivo study. Life Sci. 2007, 80, 388–396. [Google Scholar] [CrossRef]
- Dastan, T.; Turan, K. (17) In Vitro Characterization and Delivery of Chitosan-DNA Microparticle Intomammalian Cells|Request PDF. Available online: https://www.researchgate.net/publication/8348095_In_vitro_characterization_and_delivery_of_chitosan-DNA_microparticle_intomammalian_cells/citations (accessed on 14 October 2022).
- Dastan, T.; Turan, K. In Vitro Characterization and Delivery of Chitosan-DNA Microparticles into Mammalian Cells. Available online: https://pubmed.ncbi.nlm.nih.gov/15367377/ (accessed on 30 September 2022).
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-loaded chitosan nanospheres from nano-emulsion templating for the topical treatment of herpesviruses infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Sakono, N.; Nakamura, K.; Ohshima, T.; Hayakawa, R.; Sakono, M. Tyrosinase-mediated Peptide Conjugation with Chitosan-coated Gold Nanoparticles. Anal. Sci. 2019, 35, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Vyas, R.; Gupta, N.; Nimesh, S. Chitosan nanoparticles for efficient and targeted delivery of anticancer drugs. In Nanobiomaterials in Cancer Therapy: Applications of Nanobiomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 281–306. [Google Scholar]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.A.; Madni, A.; Rehman, M.; Rahim, M.A.; Jabar, A. Ionically cross-linked chitosan nanoparticles for sustained delivery of docetaxel: Fabrication, post-formulation and acute oral toxicity evaluation. Int. J. Nanomed. 2019, 14, 10035–10046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-C.; Mi, F.-L.; Liao, Z.-X.; Hsiao, C.-W.; Sonaje, K.; Chung, M.-F.; Hsu, L.-W.; Sung, H.-W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Rezende, C.M.F.; Silva, M.R.; Borges, O.M.; Pêgo, A.P.; Goes, A.M. Oral vaccination based on DNA-chitosan nanoparticles against schistosoma mansoni infection. Sci. World J. 2012, 2012, 938457. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Administration Pathway | Advantages | Disadvantages | |
|---|---|---|---|
| Enteral | Oral | Presents the greatest comfort for the patient and the most economical. The effect can be local or systemic Easy access. Formulations can be created that protect the active ingredients from degradation. | Impossible to use in uncooperative patients with vomiting or loss of consciousness. Unpredictable absorption due to the first pass liver effect. In the case of stomach or bowel surgery, it is not an option. |
| Rectal | Excellent absorption through hemorrhoidal veins that connect directly into the vena cava. Prevents the effect of the first liver past. | It is an unpleasant way for patients. It cannot be used after surgery. | |
| Parenteral | Subcutaneous | They are the first choice for active ingredients with low bioavailability in oral forms. Rapid absorption and effect. Depending on the formulation, they can have a prolonged effect. | Absorption becomes irregular in poorly perfused areas or organs. Small volumes. Irritating substances cannot be administered. |
| Intramuscular | Absorption and immediate effect. The first choice for drugs with low bioavailability orally or when the GI tract is compromised. | Painful A bad application can imply paralysis or muscular atrophy. | |
| Intravenous | Dependable and reproducible effects. The full dose enters the systemic circulation, allowing an immediate response. | Requires special equipment or trained personnel. It is invasive and painful. The devices as well as the contact area are susceptible to infection. There is no way to withdraw the drug in case of adverse reactions. | |
| Topic | Non-invasive and easy to administer. Painless. Local effect. Patient satisfaction levels are high. | Molecular way and low-fat solubility are determining factors for its absorption. Low absorption. Skin irritation. | |
| Inhale | Immediate absorption thanks to the large surface area of the respiratory tracts. | Bioavailability depends on the patient’s inhalation technique and the size of the molecule. |
| Advantages | Disadvantages |
|---|---|
| Simple to use and easy to access; it is convenient for the patient. | Patient cooperation is required. |
| It is a safe and practical way. | Absorption cannot be predicted due to irregular release. |
| In case of adverse reaction, it can be removed by physical means such as vomiting or gastric lavage. | Gastric mucosa irritation. |
| It is an economical and effective method. | The taste of some medicines can be unpleasant. |
| Does not require trained personnel or training. | In extremely apprehensive patient it is not useful. |
| The effect can be prolonged beyond the therapeutic period. | The effect can be prolonged beyond the therapeutic period. |
| Physicochemical Barriers | Biopharmaceutical Barriers | Physiologic Barriers | Clinic Barriers |
|---|---|---|---|
| Low solubility. | Low permeability. | Presence of mucus. | Fed- and fasted-state variability in drug absorption. |
| pH dependent solubility or degradation. | Degradation in the GI tract. | Differences in the pH of the GI tract (pH 1.2–7.4). | Inter and intra individual differences in the oral route. |
| Extensive ionization at GI pH range. | Presence of drug efflux transporters. | Rapid gastric emptying. | Deficiencies in the permanence and emptying of the GI tract. |
| High lipophilicity. | Variation of pH and mucosal layer thickness variations in the GI tract, depending upon the location. | Gastric and intestinal motility. | Presence of diseases. |
| High molecular weight. | Effect of the firs live pass. | ||
| Presence of digestive enzymes and microbiota of the GI tract. |
| Part | pH | Extent (cm) | Mean Length (cm) | Mucus Regular Depth (µm) | Mucus Income (hour) | Surface Area (m2) |
|---|---|---|---|---|---|---|
| Stomach | 1.5–5 | 20 | NA | 245 +/− 200 | 24–48 | 0.053 |
| Duodenum | 5.0–7.0 | 17–56 | 680 | 15.5 | 252 | |
| Jejunum | 6.0–7.4 | 280–1000 | ||||
| Ileum | ||||||
| Colon | 5.5–7.0 | 80–313 | 93 | 132 +/− 25 | 0.35 | |
| Total | 835 |
| Carriers | Nanoparticles |
|---|---|
| Raw materials for massive production [52] | Raw materials for massive production [52] |
| Low cost | Limited or no use of organic solvents |
| Reduction of environmental cost [52] | Reproducibility and repeatability |
| Easy and flexible processing methods [52] | Protect drugs and other molecules with biological activity against the environment [53] |
| Non-toxic and immunogenic | Freeze-drying capacity |
| Soluble in water | Stable after administration |
| Lightweight, chemical stability, and elasticity | Biocompatibility, biodegradability and non-toxicity [54] |
| Protect drugs and molecules against the environment [53] | Bioavailability and therapeutic index [53] |
| Potential use for controlled release [53] |
| Drug | Function | Results | Autor | References |
|---|---|---|---|---|
| Pramipexole hydrochloride | Symptoms of Parkinson’s disease | Diameter 243 +/− 12–337 +/− 13 nm Zeta potential 23 +/− mV Entrapment efficiency 63% | S. Papadimitriou, D. Bikiaris, K. Avgoustakis, E. Karavas, and M. Georgarakis | [73] |
| 5-fluorouracil Tamoxifen Doxorubicin hydrochloride | Chemotherapeutic | Diameter: 283.9 +/− 5.25 nm Zeta potential 45.3 +/− 3.23 mV Encapsulation efficiency 44.28 +/− 1.69% | M. A. Mohammed et al., L. Sun et al., J. G. Rosch et al. | [34,74,75] |
| Chatechin and apigallocatechin Quercetin | Flavonoids (antioxidants) | Diameter 110–335 nm Zeta potential 30 mV | M. A. Mohammed et al., A. Dube et al., A. I. Barbosa et al., | [34,76,77] |
| Alendronate sodium | Osteoporosis treatment | Diameter 200 nm | M. A. Mohammed, J. T. M. Syeda, K. M. Wasan, and E. K. Wasan | [34] |
| Cyclosporin A | Immunosuppression | Diameter: 150 nm Zeta potential: +30 mV | M. H. El-Shabouri | [78] |
| Protein and gene delivery | Gene therapy | Diameter: 350 nm | C. Y. Wong et al., R. M. Saeed et al. | [79,80] |
| Insulin | Diabetes mellitus treatment | Diameter: 100–200 nm Encapsulation efficiency: 85% | F. Cui et al., L. Li et al. | [81,82,83,84] |
| Deferoxamine | Iron-chelating drug | Diameter: 150–400 nm | M. Lazaridou et al. | [85] |
| Aspirin Probucol | Treatment of restenosis (hypolipemic and antiplatelet agent) | H. Liu and J. He | [86] | |
| Interferon-α | Cancer treatment and antiviral activity | Diameter 200 nm Entrapment efficiency 89% | C. C. Cánepa, J. C. Imperiale, C. A. Berini, M. Lewicki, A. Sosnik, and M. M. Biglione | [87] |
| Foscarnet Tenofovir disoproxil fumarate | Antiviral agent Antiretroviral therapy (HIV) | Diameter 450 nm Zeta potential 20/25 mV Drug loading 55% | E. Russo et al., J. Shailender et al. | [88,89] |
| Heparin | Anticoagulant properties (venous thrombosis, pulmonary embolisms) | Microemulsion method Diameter 146 +/− 33 nm Zeta potential 35 mV | W. Dong et al. W. Dong et al. | [71,90] |
| Puerarin | Treatment of coronary heart disease. | Diameter 126.28 nm PDI 0.122 Encapsulation rate 94.49% | J. Yan et al. | [91] |
| Rifampicin | Antibiotic | Diameter 221.9 nm Entrapment efficiency 44.17% Drug loading 42.96% | B. K. Patel, R. H. Parikh, and P. S. Aboti | [92] |
| Curcumin diglutaric acid (CG) | Prodrug of curcumin | Diameter 345 nm Zeta potential 22.1 mV | F. N. Sorasitthiyanukarn, C. Muangnoi, P. Ratnatilaka Na Bhuket, P. Rojsitthisak, and P. Rojsitthisak | [93] |
| Liraglutide | Diabetes treatment | Diameter 100 nm Loading efficiency 92.5% Loading capacity 54.16% | F. Shamekhi, E. Tamjid, and K. Khajeh | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadarrama-Escobar, O.R.; Serrano-Castañeda, P.; Anguiano-Almazán, E.; Vázquez-Durán, A.; Peña-Juárez, M.C.; Vera-Graziano, R.; Morales-Florido, M.I.; Rodriguez-Perez, B.; Rodriguez-Cruz, I.M.; Miranda-Calderón, J.E.; et al. Chitosan Nanoparticles as Oral Drug Carriers. Int. J. Mol. Sci. 2023, 24, 4289. https://doi.org/10.3390/ijms24054289
Guadarrama-Escobar OR, Serrano-Castañeda P, Anguiano-Almazán E, Vázquez-Durán A, Peña-Juárez MC, Vera-Graziano R, Morales-Florido MI, Rodriguez-Perez B, Rodriguez-Cruz IM, Miranda-Calderón JE, et al. Chitosan Nanoparticles as Oral Drug Carriers. International Journal of Molecular Sciences. 2023; 24(5):4289. https://doi.org/10.3390/ijms24054289
Chicago/Turabian StyleGuadarrama-Escobar, Omar Rodrigo, Pablo Serrano-Castañeda, Ericka Anguiano-Almazán, Alma Vázquez-Durán, Ma. Concepción Peña-Juárez, Ricardo Vera-Graziano, Miriam Isabel Morales-Florido, Betsabe Rodriguez-Perez, Isabel Marlen Rodriguez-Cruz, Jorge Esteban Miranda-Calderón, and et al. 2023. "Chitosan Nanoparticles as Oral Drug Carriers" International Journal of Molecular Sciences 24, no. 5: 4289. https://doi.org/10.3390/ijms24054289