Charge Transfer and Electron Production in Proton Collisions with Uracil: A Classical and Semiclassical Study
Abstract
:1. Introduction
2. Results and Discussion
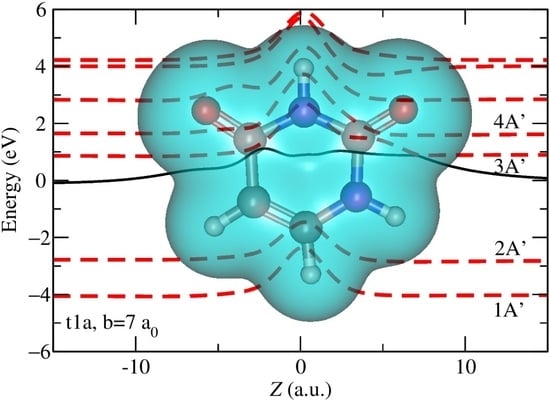
2.1. Semiclassical Results
2.2. CTMC Results
3. Materials and Methods
3.1. Semiclassical Method
3.2. CTMC Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| U | Uracil |
| CT | Charge transfer |
| EP | Electron production |
| CTMC | classical-trajectory Monte Carlo |
| MO | Molecular orbital |
| CASSCF | Complete active space self consistent-field |
| PEC | Potential energy curve |
| IPM | Independent particle model |
| EC | Entrance channel |
References
- Tabet, J.; Eden, S.; Feil, S.; Abdoul-Carime, H.; Farizon, B.; Farizon, M.; Ouaskit, S.; Märk, T.D. Absolute total and partial cross sections for ionization of nucleobases by proton impact in the Bragg peak velocity range. Phys. Rev. A 2010, 82, 022703. [Google Scholar] [CrossRef] [Green Version]
- Moretto-Capelle, P.; Le Padellec, A. Electron spectroscopy in proton collisions with dry gas-phase uracil base. Phys. Rev. A 2006, 74, 062705. [Google Scholar] [CrossRef]
- Itoh, A.; Iriki, Y.; Imai, M.; Champion, C.; Rivarola, R.D. Cross sections for ionization of uracil by MeV-energy-proton impact. Phys. Rev. A 2013, 88, 052711. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.R.; Monti, J.M.; Misra, D.; Weck, P.F.; Rivarola, R.D.; Tribedi, L.C. Electron emission from bromouracil and uracil induced by protons and radiosensitization. New J. Phys. 2022, 24, 073035. [Google Scholar] [CrossRef]
- Bacchus-Montabonel, M.C.; Łabuda, M.; Tergiman, Y.S.; Sienkiewicz, J.E. Theoretical treatment of charge-transfer processes induced by collision of Cq+ ions with uracil. Phys. Rev. A 2005, 72, 052706. [Google Scholar] [CrossRef]
- Bacchus-Montabonel, M.C.; Tergiman, Y.S. Anisotropic effect in the charge transfer of Cq+ ions with uracil. Phys. Rev. A 2006, 74, 054702. [Google Scholar] [CrossRef]
- Bacchus-Montabonel, M.C.; Tergiman, Y.S. Charge transfer dynamics of carbon ions with uracil and halouracil targets at low collision energies. Chem. Phys. Lett. 2011, 503, 45–48. [Google Scholar] [CrossRef]
- Blanco, F.; García, G. Screening corrections for calculation of electron scattering from polyatomic molecules. Phys. Lett. A 2003, 317, 458–462. [Google Scholar] [CrossRef]
- Paredes, S.; Illescas, C.; Méndez, L. On the use of additivity rules to estimate electron production cross sections in proton-biomolecule collisions. Eur. Phys. J. D 2015, 69, 178. [Google Scholar] [CrossRef] [Green Version]
- Lüdde, H.J.; Horbatsch, M.; Kirchner, T. Proton-impact-induced electron emission from biologically relevant molecules studied with a screened independent atom model. J. Phys. B At. Mol. Opt. Phys. 2019, 52, 195203. [Google Scholar] [CrossRef]
- Lüdde, H.J.; Kalkbrenner, T.; Horbatsch, M.; Kirchner, T. Nonperturbative scaling behavior for net ionization of biologically relevant molecules by multiply charged heavy-ion impact. Phys. Rev. A 2020, 101, 062709. [Google Scholar] [CrossRef]
- Lekadir, H.; Abbas, I.; Champion, C.; Fojón, O.; Rivarola, R.D.; Hanssen, J. Single-electron-loss cross sections of DNA and RNA bases impacted by energetic multicharged ions: A classical Monte Carlo approximation. Phys. Rev. A 2009, 79, 062710. [Google Scholar] [CrossRef]
- Sarkadi, L. Classical treatment of the electron emission from collisions of uracil molecules with fast protons. Phys. Rev. A 2015, 92, 062704. [Google Scholar] [CrossRef]
- Galassi, M.E.; Champion, C.; Weck, P.F.; Rivarola, R.D.; Fojón, O.; Hanssen, J. Quantum-mechanical predictions of DNA and RNA ionization by energetic proton beams. Phys. Med. Biol. 2012, 57, 2081. [Google Scholar] [CrossRef] [PubMed]
- Purkait, K.; Samaddar, S.; Purkait, M.; Jana, D. Ionization and Electron Capture Cross Sections for Single-Electron Removal from Biological Molecules by Swift Ion. Braz. J. Phys. 2021, 51, 1–12. [Google Scholar] [CrossRef]
- Covington, C.; Hartig, K.; Russakoff, A.; Kulpins, R.; Varga, K. Time-dependent density-functional-theory investigation of the collisions of protons and α particles with uracil and adenine. Phys. Rev. A 2017, 95, 052701. [Google Scholar] [CrossRef] [Green Version]
- Rabadán, I.; Méndez, L. Orientation effects in ion-molecule collisions. J. Phys. Conf. Ser. 2017, 875, 012009. [Google Scholar] [CrossRef]
- Rai, S.; Bijlsma, K.I.; Rabadán, I.; Méndez, L.; Wolff, P.A.J.; Salverda, M.; Versolato, O.O.; Hoekstra, R. Charge exchange in collisions of 1–100-keV Sn3+ ions with H2 and D2. Phys. Rev. A 2022, 106, 012804. [Google Scholar] [CrossRef]
- Willis, S.L.; Peach, G.; McDowell, M.R.C.; Banerji, J. Charge transfer and ionisation processes in collisions involving atoms and ions of hydrogen and helium. J. Phys. B At. Mol. Phys. 1985, 18, 3939. [Google Scholar] [CrossRef]
- Lüdde, H.J.; Horbatsch, M.; Kirchner, T. Electron capture and ionization cross-section calculations for proton collisions with methane and the DNA and RNA nucleobases. Eur. Phys. J. D 2019, 73, 249. [Google Scholar] [CrossRef]
- Shukla, M.K.; Leszczynski, J. Tautomerism in nucleic acid bases and base pairs: A brief overview. WIREs Comput. Mol. Sci. 2013, 3, 637–649. [Google Scholar] [CrossRef]
- Schneiderman, S.B.; Russek, A. Velocity-Dependent Orbitals in Proton-On-Hydrogen-Atom Collisions. Phys. Rev. 1969, 181, 311–321. [Google Scholar] [CrossRef]
- Errea, L.F.; Gorfinkiel, J.D.; Macías, A.; Méndez, L.; Riera, A. Implementation of the sudden approximation eikonal method in ion - diatom collisions. J. Phys. B At. Mol. Opt. Phys. 1997, 30, 3855. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M. Molpro: A general-purpose quantum chemistry program package. WIREs Comput. Mol. Sci. 2012, 2, 242–253. [Google Scholar] [CrossRef]
- Widmark, P.O.; Malmqvist, P.Å.; Roos, B.O. Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions. Theor. Chim. Acta 1990, 77, 291–306. [Google Scholar] [CrossRef]
- Errea, L.F.; Mendez, L.; Riera, A. On the choice of translation factors for approximate molecular wavefunctions. J. Phys. B At. Mol. Phys. 1982, 15, 101. [Google Scholar] [CrossRef]
- Abrines, R.; Percival, I.C. Classical theory of charge transfer and ionization of hydrogen atoms by protons. Proc. Phys. Soc. 1966, 88, 861. [Google Scholar] [CrossRef]
- Errea, L.F.; Illescas, C.; Méndez, L.; Pons, B.; Rabadán, I.; Riera, A. Classical calculation of ionization and electron-capture total cross sections in H++H2O collisions. Phys. Rev. A 2007, 76, 040701. [Google Scholar] [CrossRef]
- Hardie, D.J.W.; Olson, R.E. Charge transfer and ionisation processes involving multiply charged ions in collision with atomic hydrogen. J. Phys. B At. Mol. Phys. 1983, 16, 1983. [Google Scholar] [CrossRef]
- Illescas, C.; Riera, A. Classical study of single-electron capture and ionization processes in Aq++(H,H2) collisions. Phys. Rev. A 1999, 60, 4546–4560. [Google Scholar] [CrossRef]
- Kirchner, T.; Gulyás, L.; Lüdde, H.J.; Engel, E.; Dreizler, R.M. Influence of electronic exchange on single and multiple processes in collisions between bare ions and noble-gas atoms. Phys. Rev. A 1998, 58, 2063–2076. [Google Scholar] [CrossRef]









| Electronic State | Dominant Configuration | Energy (eV) |
|---|---|---|
| 1A | 0.0 | |
| 1A | 0.75 | |
| 2A | 1.40 | |
| 2A | 2.95 | |
| 3A | 4.28 | |
| 4A | 4.47 | |
| 3A | 4.91 | |
| 4A | 6.22 |
| MO (A) | MO (A) | ||
|---|---|---|---|
| 24 | 5 | ||
| 23 | 4 | ||
| 22 | 3 | ||
| 21 | 2 | ||
| 20 | 1 | ||
| 19 | 0.6860 | ||
| 18 | 0.7224 | ||
| 17 | 0.7742 | ||
| 16 | 0.8153 | ||
| 15 | 0.9109 | ||
| 14 | 0.9416 | ||
| 13 | 1.0954 | ||
| 12 | 1.2528 | ||
| 11 | 1.3211 | ||
| 10 | 1.4068 | ||
| 9 | 1.4442 |
| Electron Production | Charge Transfer | |||
|---|---|---|---|---|
| E (keV) | a | b | a | b |
| 20 | ||||
| 30 | ||||
| 50 | ||||
| 100 | ||||
| 225 | ||||
| 500 | ||||
| 1000 | ||||
| 2000 | ||||
| 2500 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illescas, C.; Méndez, L.; Bernedo, S.; Rabadán, I. Charge Transfer and Electron Production in Proton Collisions with Uracil: A Classical and Semiclassical Study. Int. J. Mol. Sci. 2023, 24, 2172. https://doi.org/10.3390/ijms24032172
Illescas C, Méndez L, Bernedo S, Rabadán I. Charge Transfer and Electron Production in Proton Collisions with Uracil: A Classical and Semiclassical Study. International Journal of Molecular Sciences. 2023; 24(3):2172. https://doi.org/10.3390/ijms24032172
Chicago/Turabian StyleIllescas, Clara, Luis Méndez, Santiago Bernedo, and Ismanuel Rabadán. 2023. "Charge Transfer and Electron Production in Proton Collisions with Uracil: A Classical and Semiclassical Study" International Journal of Molecular Sciences 24, no. 3: 2172. https://doi.org/10.3390/ijms24032172