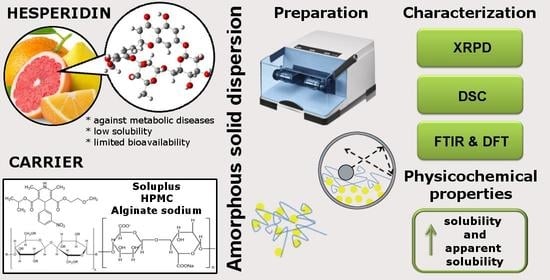
Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. The Active Substance and Materials
4.2. Preparation of Amorphous Hesperidin and Hesperidin-Carrier Amorphous Solid Dispersions
4.3. The Characterization of Hesperidin-Carrier Amorphous Solid Dispersions
4.3.1. X-ray Powder Diffraction (XRPD)
4.3.2. Thermogravimetric Analysis (TG)
4.3.3. Differential Scanning Calorimetry (DSC) and Gordon–Taylor equation
4.3.4. Fourier-Transform Infrared Spectroscopy (FTIR) and Density Functional Theory (DFT) Calculations
4.4. Solubility and Apparent Solubility Studies of Hesperidin and Its Amorphous Solid Dispersions with Carriers
4.4.1. HPLC Method
4.4.2. Media for Solubility and Apparent Solubility Studies
4.4.3. Procedure of Solubility Studies
4.4.4. Procedure of Apparent Solubility Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Siedlecka-Kroplewska, K.; Kmiec, Z.; Gorska-Ponikowska, M. Modulation of Autophagy in Cancer Cells by Dietary Polyphenols. Antioxidants 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Kamaly, O.M.; Jawhari, F.Z.; Imtara, H.; Grafov, A.; Bousta, D. The Potential of Parsley Polyphenols and Their Antioxidant Capacity to Help in the Treatment of Depression and Anxiety: An In Vivo Subacute Study. Molecules 2021, 26, 2009. [Google Scholar] [CrossRef] [PubMed]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Shi, X.; Fan, N.; Zhang, G.; Sun, J.; He, Z.; Li, J. Quercetin amorphous solid dispersions prepared by hot melt extrusion with enhanced solubility and intestinal absorption. Pharm. Dev. Technol. 2020, 25, 472–481. [Google Scholar] [CrossRef]
- Kuntic, V.; Brboric, J.; Holclajtner-Antunovic, I.; Uskokovic-Markovic, S. Evaluating the bioactive effects of flavonoid hesperidin: A new literature data survey. Vojnosanit. Pregl. 2014, 71, 60–65. [Google Scholar] [CrossRef]
- ur Rehman, M.F.; Batool, A.I.; Qadir, R.; Aslam, M. Hesperidin and naringenin. In A centum of Valuable Plant Bioactives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 403–444. [Google Scholar]
- Li, Y.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2019, 105, 77–85. [Google Scholar] [CrossRef]
- Cicero-Sarmiento, C.G.; Ortiz-Andrade, R.; Araujo-León, J.A.; Segura-Campos, M.R.; Vazquez-Garcia, P.; Rubio-Zapata, H.; Hernández-Baltazar, E.; Yañez-Pérez, V.; Sánchez-Recillas, A.; Sánchez-Salgado, J.C. Preclinical Safety Profile of an Oral Naringenin/Hesperidin Dosage Form by In Vivo Toxicological Tests. Sci. Pharm. 2022, 90, 28. [Google Scholar] [CrossRef]
- Haidari, F.; Heybar, H.; Jalali, M.T.; Ahmadi Engali, K.; Helli, B.; Shirbeigi, E. Hesperidin supplementation modulates inflammatory responses following myocardial infarction. J. Am. Coll. Nutr. 2015, 34, 205–211. [Google Scholar] [CrossRef]
- Giannini, I.; Amato, A.; Basso, L.; Tricomi, N.; Marranci, M.; Pecorella, G.; Tafuri, S.; Pennisi, D.; Altomare, D.F. Flavonoids mixture (diosmin, troxerutin, hesperidin) in the treatment of acute hemorrhoidal disease: A prospective, randomized, triple-blind, controlled trial. Tech. Coloproctol. 2015, 19, 339–345. [Google Scholar] [CrossRef]
- Salden, B.N.; Troost, F.J.; de Groot, E.; Stevens, Y.R.; Garcés-Rimón, M.; Possemiers, S.; Winkens, B.; Masclee, A.A. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am. J. Clin. Nutr. 2016, 104, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Hesperidin supplementation alleviates oxidative DNA damage and lipid peroxidation in type 2 diabetes: A randomized double-blind placebo-controlled clinical trial. Phyther. Res. 2017, 31, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Li, G.; Du, W.; Zhang, J.; Li, W.; Xu, J.; Hu, M.; Zhu, Z. In Vivo Pharmacokinetics of Hesperidin Are Affected by Treatment with Glucosidase-like BglA Protein Isolated from Yeasts. J. Agric. Food Chem. 2008, 56, 5550–5557. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Teng, J.; Selbo, J. Amorphous solid dispersion of epigallocatechin gallate for enhanced physical stability and controlled release. Pharmaceuticals 2017, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Gilley, A.D.; Arca, H.C.; Nichols, B.L.B.; Mosquera-Giraldo, L.I.; Taylor, L.S.; Edgar, K.J.; Neilson, A.P. Novel cellulose-based amorphous solid dispersions enhance quercetin solution concentrations in vitro. Carbohydr. Polym. 2017, 157, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Wegiel, L.A.; Zhao, Y.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Curcumin amorphous solid dispersions: The influence of intra and intermolecular bonding on physical stability. Pharm. Dev. Technol. 2014, 19, 976–986. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Ivanisevic, I.; Bates, S.; Alonzo, D.E.; Taylor, L.S. Evaluation of Drug-Polymer Miscibility in Amorphous Solid Dispersion Systems. Pharm. Res. 2009, 26, 2523–2534. [Google Scholar] [CrossRef]
- Wei, Q.; Keck, C.M.; Müller, R.H. Oral hesperidin—Amorphization and improved dissolution properties by controlled loading onto porous silica. Int. J. Pharm. 2017, 518, 253–263. [Google Scholar] [CrossRef]
- Wei, Q.; Keck, C.M.; Müller, R.H. CapsMorph® technology for oral delivery–theory, preparation and characterization. Int. J. Pharm. 2015, 482, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Srirangam, R. Solubility, Stability, Physicochemical Characteristics and In Vitro Ocular Tissue Permeability of Hesperidin: A Natural Bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalita, B.; Patwary, B.N. Formulation and In Vitro Evaluation of Hesperidin-Phospholipid Complex and its Antioxidant Potential. Curr. Drug Ther. 2020, 15, 28–36. [Google Scholar] [CrossRef]
- Stanisic, D.; Liu, L.H.B.; dos Santos, R.V.; Costa, A.F.; Durán, N.; Tasic, L. New Sustainable Process for Hesperidin Isolation and Anti-Ageing Effects of Hesperidin Nanocrystals. Molecules 2020, 25, 4534. [Google Scholar] [CrossRef] [PubMed]
- Omidfar, F.; Gheybi, F.; Davoodi, J.; Amirinejad, M.; Badiee, A. Nanophytosomes of hesperidin and of hesperetin: Preparation, characterization, and in vivo evaluation. Biotechnol. Appl. Biochem. 2022. [Google Scholar] [CrossRef]
- Joshi, S.; Dhingra, A.K.; Chopra, B.; Dass, R.; Guarve, K.; Sapra, S. Formulation and Evaluation of Solid Dispersions of Poorly Water-Soluble Drug-Hesperidin. Lett. Appl. NanoBioScience 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. J. Appl. Polym. Sci. 2006, 102, 460–471. [Google Scholar] [CrossRef]
- Elghani, E.M.A.; Omar, F.A.; Emam, M.M.A.-A.; Al-Mahallawi, A.M.; Tadros, S.H.; Soliman, F.M.; ElSayed, A.M. Hesperidin hexosomal loaded nanodispersion: Insights of its antimycobacterial, cytotoxic and anti-HCoV effects. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, Z.; Lan, Q.; Zhou, H.; Mai, Z.; Wang, Y.; Ding, X.; Zhang, W.; Pi, J.; Evans, C.E. Nasal delivery of hesperidin/chitosan nanoparticles suppresses cytokine storm syndrome in a mouse model of acute lung injury. Front. Pharmacol. 2021, 11, 592238. [Google Scholar] [CrossRef] [PubMed]
- Jangde, R.; Elhassan, G.O.; Khute, S.; Singh, D.; Singh, M.; Sahu, R.K.; Khan, J. Hesperidin-Loaded Lipid Polymer Hybrid Nanoparticles for Topical Delivery of Bioactive Drugs. Pharmaceuticals 2022, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef]
- Kaushal, A.M.; Gupta, P.; Bansal, A.K. Amorphous drug delivery systems: Molecular aspects, design, and performance. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21. [Google Scholar] [CrossRef]
- Thakral, N.K.; Ray, A.R.; Bar-Shalom, D.; Eriksson, A.H.; Majumdar, D.K. Soluplus-Solubilized Citrated Camptothecin—A Potential Drug Delivery Strategy in Colon Cancer. AAPS PharmSciTech 2012, 13, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Huang, W.; Chen, J.; Wang, H.; Zhang, S.; Xiong, S. The Synergetic Effects of Nonpolar and Polar Protic Solvents on the Properties of Felodipine and Soluplus in Solutions, Casting Films, and Spray-Dried Solid Dispersions. J. Pharm. Sci. 2018, 107, 1615–1623. [Google Scholar] [CrossRef]
- Lan, Y.; Ali, S.; Langley, N. Characterization of Soluplus by FTIR and Raman Spectroscopy; BASF Corporation, Pharma Ingredients and Services: Tarrytown, NY, USA, 2010. [Google Scholar] [CrossRef]
- Patnaik, S.; Aditha, S.K.; Rattan, T.; Kamisetti, V. Aceclofenac-Soluplus® Nanocomposites for Increased Bioavailability. Soft Nanosci. Lett. 2015, 5, 13–20. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Neau, S.H. Investigation of the in vitro performance difference of drug-Soluplus® and drug-PEG 6000 dispersions when prepared using spray drying or lyophilization. Saudi Pharm. J. 2017, 25, 419–439. [Google Scholar] [CrossRef] [Green Version]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Kumar Trivedi, M.; Tallapragada, R.M. Characterization of Physicochemical and Thermal Properties of Chitosan and Sodium Alginate after Biofield Treatment. Pharm. Anal. Acta 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Otoni, C.G.; Lorevice, M.V.; de Moura, M.R.; Mattoso, L.H.C. On the effects of hydroxyl substitution degree and molecular weight on mechanical and water barrier properties of hydroxypropyl methylcellulose films. Carbohydr. Polym. 2018, 185, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, L.; Ahmad, M.; Usman, M. Evaluation of cross-linked hydroxypropyl methylcellulose graft-methacrylic acid copolymer as extended release oral drug carrier. Cell. Chem. Technol 2015, 49, 143–151. [Google Scholar]
- Hay, W.T.; Fanta, G.F.; Peterson, S.C.; Thomas, A.J.; Utt, K.D.; Walsh, K.A.; Boddu, V.M.; Selling, G.W. Improved hydroxypropyl methylcellulose (HPMC) films through incorporation of amylose-sodium palmitate inclusion complexes. Carbohydr. Polym. 2018, 188, 76–84. [Google Scholar] [CrossRef]
- Akinosho, H.; Hawkins, S.; Wicker, L. Hydroxypropyl methylcellulose substituent analysis and rheological properties. Carbohydr. Polym. 2013, 98, 276–281. [Google Scholar] [CrossRef]
- Langkilde, F.W.; Svantesson, A. Identification of celluloses with Fourier-Transform (FT) mid-infrared, FT-Raman and near-infrared spectrometry. J. Pharm. Biomed. Anal. 1995, 13, 409–414. [Google Scholar] [CrossRef]
- Gustafsson, C.; Nyström, C.; Lennholm, H.; Bonferoni, M.C.; Caramella, C.M. Characteristics of Hydroxypropyl Methylcellulose Influencing Compactibility and Prediction of Particle and Tablet Properties by Infrared Spectroscopy. J. Pharm. Sci. 2003, 92, 494–504. [Google Scholar] [CrossRef]
- Shi, S.-C.; Wu, J.-Y.; Huang, T.-F. Raman, FTIR, and XRD study of MoS2 enhanced hydroxypropyl methylcellulose green lubricant. Opt. Quantum Electron. 2016, 48, 474. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chen, L.-C.; Lin, S.-B.; Chen, H.-H. Nano-biomaterials application: In situ modification of bacterial cellulose structure by adding HPMC during fermentation. Carbohydr. Polym. 2011, 83, 979–987. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Thakral, F.; Singhal, P.; Aggarwal, D.; Srivastava, S.; Pandey, A.; Sak, K.; Varol, M.; Khan, M.A.; et al. Molecular mechanisms of action of hesperidin in cancer: Recent trends and advancements. Exp. Biol. Med. 2020, 245, 486–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J. Therm. Anal. Calorim. 2006, 83, 283–290. [Google Scholar] [CrossRef]
- Yen, K.; Mandal, T.K.; Woo, E.M. Enhancement of bio-compatibility via specific interactions in polyesters modified with a bio-resourceful macromolecular ester containing polyphenol groups. J. Biomed. Mater. Res. Part A 2008, 86, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-T.; Wu, M.-C.; Lee, M.-H. Novel miscible blends of biodegradable polymer and biocompatible polyphenol acquired from natural source. J. Polym. Res. 2013, 20, 1–11. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, J.; Chang, J.; Liu, X.; Xue, H.; Wang, R.; Li, Z.; Li, C.; Wang, J.; Liu, C. Soluplus-mediated diosgenin amorphous solid dispersion with high solubility and high stability: Development, characterization and oral bioavailability. Drug Des. Dev. Ther. 2020, 14, 2959. [Google Scholar] [CrossRef]
- Basha, M.; Salama, A.; Noshi, S.H. Soluplus® based solid dispersion as fast disintegrating tablets: A combined experimental approach for enhancing the dissolution and antiulcer efficacy of famotidine. Drug Dev. Ind. Pharm. 2020, 46, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Salah, I.; Shamat, M.A.; Cook, M.T. Soluplus solutions as thermothickening materials for topical drug delivery. J. Appl. Polym. Sci. 2019, 136, 46915. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef]
- Nair, A.B.; Sreeharsha, N.; Al-Dhubiab, B.E.; Hiremath, J.G.; Shinu, P.; Attimarad, M.; Venugopala, K.N.; Mutahar, M. HPMC-and PLGA-based nanoparticles for the mucoadhesive delivery of Sitagliptin: Optimization and in vivo evaluation in rats. Materials 2019, 12, 4239. [Google Scholar] [CrossRef]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.B.; Rathi, S.; Jyothi, V.G.S.S.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J. Pharm. Sci. 2019, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Navarro, M.; Vallejo, F.; Borrego, F.; Tomás-Barberán, F.A. Encapsulation and Micronization Effectively Improve Orange Beverage Flavanone Bioavailability in Humans. J. Agric. Food Chem. 2014, 62, 9458–9462. [Google Scholar] [CrossRef] [PubMed]
- Talaczyńska, A.; Lewandowska, K.; Garbacki, P.; Zalewski, P.; Skibiński, R.; Miklaszewski, A.; Mizera, M.; Cielecka-Piontek, J. Solid-state stability studies of crystal form of tebipenem. Drug Dev. Ind. Pharm. 2016, 42, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Heinzerling, O.; Degenhardt, M.; Sadowski, G. Long-Term Physical Stability of PVP- and PVPVA-Amorphous Solid Dispersions. Mol. Pharm. 2017, 14, 157–171. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Lu, W.; Park, J.-B.; Morott, J.T.; Alsulays, B.B.; Majumdar, S.; Langley, N.; Kolter, K.; Gryczke, A.; Repka, M.A. Stability-enhanced Hot-melt Extruded Amorphous Solid Dispersions via Combinations of Soluplus® and HPMCAS-HF. AAPS PharmSciTech 2015, 16, 824–834. [Google Scholar] [CrossRef] [Green Version]
- Prasad, D.; Chauhan, H.; Atef, E. Amorphous Stabilization and Dissolution Enhancement of Amorphous Ternary Solid Dispersions: Combination of Polymers Showing Drug–Polymer Interaction for Synergistic Effects. J. Pharm. Sci. 2014, 103, 3511–3523. [Google Scholar] [CrossRef]











| Mass (mg) | ΔCp (J·(g K)−1) | Tg, calc (°C) | Tg, exp (°C) | Deviation | |
|---|---|---|---|---|---|
| Hes | 10.24 | 0.431 | − | 107.10 ± 0.65 | − |
| Sol | 9.45 | 0.247 | − | 79.20 ± 0.55 | − |
| Hes:Sol 1:2 | 9.28 | 0.192 | 84.52 ± 0.58 | 88.70 ± 0.86 | + |
| Hes:Sol 1:5 | 8.79 | 0.171 | 81.60 ± 0.56 | 81.30 ± 0.36 | * |
| HPMC | 6.43 | 0.211 | − | 134.20 ± 0.93 | − |
| Hes:HPMC 1:2 | 10.74 | 0.105 | 125.02 ± 0.33 | 114.30 ± 0.67 | − |
| Hes:HPMC 1:5 | 10.23 | 0.239 | 129.59 ± 0.61 | 122.10 ± 0.76 | − |
| Medium | System | Concentration [mg mL−1] | Improved Solubility [–Fold] |
|---|---|---|---|
| Water | Hesperidin | 0.009 ± 0.001 | – |
| Hes:Sol 1:2 | 1.149 ± 0.011 | 128 | |
| Hes:Sol 1:5 | 2.710 ± 0.004 | 301 | |
| Hes:SA 1:2 | 1.059 ± 0.008 | 118 | |
| Hes:SA 1:5 | 1.184 ± 0.003 | 132 | |
| Hes:HPMC 1:2 | 0.088 ± 0.001 | 10 | |
| Hes:HPMC 1:5 | 0.622 ± 0.002 | 69 | |
| phosphate buffer (pH 6.8) | Hesperidin | 0.009 ± 0.002 | – |
| Hes:Sol 1:2 | 0.869 ± 0.020 | 97 | |
| Hes:Sol 1:5 | 2.616 ± 0.036 | 291 | |
| Hes:SA 1:2 | 1.605 ± 0.014 | 178 | |
| Hes:SA 1:5 | 1.726 ± 0.003 | 192 | |
| Hes:HPMC 1:2 | 0.068 ± 0.001 | 8 | |
| Hes:HPMC 1:5 | 0.711 ± 0.007 | 79 | |
| HCl 0.1 N | Hesperidin | 0.008 ± 0.003 | – |
| Hes:Sol 1:2 | 0.777 ± 0.009 | 97 | |
| Hes:Sol 1:5 | 2.486 ± 0.033 | 311 | |
| Hes:SA 1:2 | 0.055 ± 0.002 | 7 | |
| Hes:SA 1:5 | 0.027 ± 0.002 | 4 | |
| Hes:HPMC 1:2 | 0.064 ± 0.001 | 8 | |
| Hes:HPMC 1:5 | 0.075 ± 0.001 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiak, N.; Wdowiak, K.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases. Int. J. Mol. Sci. 2022, 23, 15198. https://doi.org/10.3390/ijms232315198
Rosiak N, Wdowiak K, Tykarska E, Cielecka-Piontek J. Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases. International Journal of Molecular Sciences. 2022; 23(23):15198. https://doi.org/10.3390/ijms232315198
Chicago/Turabian StyleRosiak, Natalia, Kamil Wdowiak, Ewa Tykarska, and Judyta Cielecka-Piontek. 2022. "Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases" International Journal of Molecular Sciences 23, no. 23: 15198. https://doi.org/10.3390/ijms232315198