Thermal-Induced Percolation Phenomena and Elasticity of Highly Oriented Electrospun Conductive Nanofibrous Biocomposites for Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussions
2.1. Aligned Conductive Nanofibers Using Electrospinning
2.2. Percolation Phenomena in Oriented Conductive Nanofibrous Biocomposites
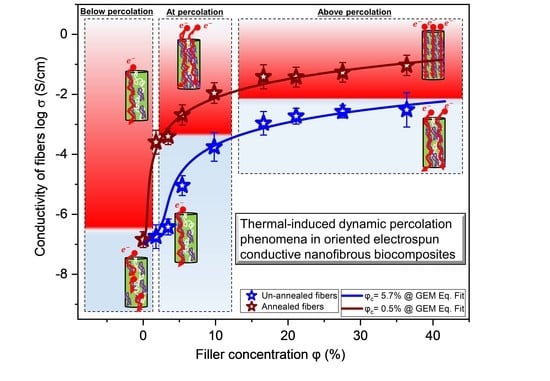
2.3. Dependence of Percolation on Annealing Temperature and Time
2.4. Percolation Reveals Time-Temperature Superposition in Conductive Nanofibers
2.5. Dependence of Crystallinity on Annealing within Confined Fiber Geometry
2.6. Dependence of Fiber Elasticity on Filler Fraction and Annealing
2.7. Conductivity in Tissue Engineering
2.8. Elasticity in Tissue Engineering
2.9. Biocompatibility of PANi-Based Conductive Fibers
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jeong, S.i.; Jun, I.D.; Choi, M.J.; Nho, Y.C.; Lee, Y.M.; Shin, H. Development of electroactive and elastic nanofibers that contain polyaniline and poly(L-lactide-co-epsilon-caprolactone) for the control of cell adhesion. Macromol. Biosci. 2008, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-W.; Bai, M.-Y.; Chang, Y.; Chung, M.-F.; Lee, T.-Y.; Wu, C.-T.; Maiti, B.; Liao, Z.-X.; Li, R.-K.; Sung, H.-W. Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials 2013, 34, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-C.; Sun, Y.-C.; Chen, Y.-H. 1 Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Vogt, L.; Greber, B.; Diecke, S.; Boccaccini, A.R.; Scheibel, T.; Engel, F.B. Electroconductive Biohybrid Hydrogel for Enhanced Maturation and Beating Properties of Engineered Cardiac Tissues. Adv. Funct. Mater. 2018, 28, 1803951. [Google Scholar] [CrossRef] [Green Version]
- Ul Haq, A.; Carotenuto, F.; de Matteis, F.; Prosposito, P.; Francini, R.; Teodori, L.; Pasquo, A.; Di Nardo, P. Intrinsically Conductive Polymers for Striated Cardiac Muscle Repair. Int. J. Mol. Sci. 2021, 22, 8550. [Google Scholar] [CrossRef]
- Goetzke, R.; Sechi, A.; de Laporte, L.; Neuss, S.; Wagner, W. Why the impact of mechanical stimuli on stem cells remains a challenge. Cell. Mol. Life Sci. 2018, 75, 3297–3312. [Google Scholar] [CrossRef]
- Akhtar, R.; Sherratt, M.J.; Cruickshank, J.K.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, C.; Li, J.; Zhou, P.; Chen, M.; Yang, H.; Li, B. The effect of the fibre orientation of electrospun scaffolds on the matrix production of rabbit annulus fibrosus-derived stem cells. Bone Res. 2015, 3, 15012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ji, Y.; Ghosh, K.; Clark, R.A.F.; Huang, L.; Rafailovich, M.H. Effects of fiber orientation and diameter on the behavior of human dermal fibroblasts on electrospun PMMA scaffolds. J. Biomed. Mater. Res. 2009, 90A, 1092–1106. [Google Scholar] [CrossRef]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef]
- Bashur, C.A.; Shaffer, R.D.; Dahlgren, L.A.; Guelcher, S.A.; Goldstein, A.S. Effect of Fiber Diameter and Alignment of Electrospun Polyurethane Meshes on Mesenchymal Progenitor Cells. Tissue Eng. Part A 2009, 15, 2435–2445. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Zhang, F.; Wang, J.-G.; Chen, Q.-C.; Yang, W.-Z.; He, N.; Jiang, Y.-Y.; Chen, F.; Liu, J.-J. Electrospinning of PELA/PPY Fibrous Conduits:Promoting Peripheral Nerve Regeneration in Rats by Self-Originated Electrical Stimulation. ACS Biomater. Sci. Eng. 2016, 2, 1572–1581. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Wei, Y. One-Dimensional Composite Nanomaterials: Synthesis by Electrospinning and Their Applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Zucolotto, V.; de Queiroz, A.A. Electrospinning of Hyperbranched Poly-L-Lysine/Polyaniline Nanofibers for Application in Cardiac Tissue Engineering. J. Macromol. Sci. Part A 2010, 47, 1203–1207. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Ramakrishna, S. Electrospun Composite Nanofibers for Tissue Regeneration. J. Nanosci. Nanotechnol. 2011, 11, 3039–3057. [Google Scholar] [CrossRef]
- Lee, Y.; Chang, C.; Yau, S.; Fan, L.; Yang, Y.; Yang, L.O.; Itaya, K. Conformations of Polyaniline Molecules Adsorbed on Au(111) Probed by in Situ STM and ex Situ XPS and NEXAFS. J. Am. Chem. Soc. 2009, 131, 6468–6474. [Google Scholar] [CrossRef]
- Lodha, A.; Kilbey II, S.M.; Ramamurthy, P.C.; Gregory, R.V. Effect of annealing on electrical conductivity and morphology of polyaniline films. J. Appl. Polym. Sci. 2001, 82, 3602–3610. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Munawar, M.A.; Schubert, D.W. Revealing Electrical and Mechanical Performances of Highly Oriented Electrospun Conductive Nanofibers of Biopolymers with Tunable Diameter. Int. J. Mol. Sci. 2021, 22, 10295. [Google Scholar] [CrossRef] [PubMed]
- Munawar, M.A.; Schubert, D.W. Highly Oriented Electrospun Conductive Nanofibers of Biodegradable Polymers-Revealing the Electrical Percolation Thresholds. ACS Appl. Polym. Mater. 2021, 3, 2889–2901. [Google Scholar] [CrossRef]
- Cao, Q.; Song, Y.; Tan, Y.; Zheng, Q. Thermal-induced percolation in high-density polyethylene/carbon black composites. Polymer 2009, 50, 6350–6356. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Ali Mohsin, M.E.; Shaikh, H.; Anis, A.; Pulose, A.M.; Yadav, M.K.; Qua, E.H.P.; Al-Zahrani, S.M. A review on electrically conductive polypropylene and polyethylene. Polym. Compos. 2014, 35, 900–914. [Google Scholar] [CrossRef]
- Spruiell, J.E. A Review of the Measurement and Development of Crystallinity and Its Relation to Properties in Neat Poly(Phenylene Sulfide) and Its Fiber Reinforced Composites. Available online: https://www.osti.gov/biblio/885940 (accessed on 1 January 2005).
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part B Rev. 2012, 19, 48–57. [Google Scholar] [CrossRef]
- Schubert, D.W. Revealing Novel Power Laws and Quantization in Electrospinning Considering Jet Splitting—Toward Predicting Fiber Diameter and Its Distribution. Macromol. Theory Simul. 2019, 28, 1900006. [Google Scholar] [CrossRef]
- Schubert, D.W.; Allen, V.; Dippel, U. Revealing Novel Power Laws and Quantization in Electrospinning Considering Jet Splitting—Toward Predicting Fiber Diameter and Its Distribution Part II Experimental. Adv. Eng. Mater. 2021, 23, 2001161. [Google Scholar] [CrossRef]
- McLachlan, D.S.; Blaszkiewicz, M.; Newnham, R.E. Electrical Resistivity of Composites. J. Am. Ceram. Soc. 1990, 73, 2187–2203. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574–588. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Zhao, X.; Wu, L.; Zhang, Q. Graphene-Wrapped Polyaniline Nanowire Arrays on Nitrogen-Doped Carbon Fabric as Novel Flexible Hybrid Electrode Materials for High-Performance Supercapacitor. Langmuir 2014, 30, 5306–5313. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C. Highly conductive and flexible silk fabric via electrostatic self assemble between reduced graphene oxide and polyaniline. Org. Electron. 2018, 55, 26–34. [Google Scholar] [CrossRef]
- Huang, T.-M.; Batra, S.; Hu, J.; Miyoshi, T.; Cakmak, M. Chemical cross-linking of conducting poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) (PEDOT:PSS) using poly(ethylene oxide) (PEO). Polymer 2013, 54, 6455–6462. [Google Scholar] [CrossRef]
- Massaglia, G.; Chiodoni, A.; Marasso, S.L.; Pirri, C.F.; Quaglio, M.; Tan, B. Electrical Conductivity Modulation of Crosslinked Composite Nanofibers Based on PEO and PEDOT:PSS. J. Nanomater. 2018, 2018, 3286901. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, R.; Rodriguez, V.; Ceylan, M. Enhancing thermal and ionic conductivities of electrospun PAN and PMMA nanofibers by graphene nanoflake additions for battery-separator applications. Int. J. Energy Res. 2014, 38, 2044–2051. [Google Scholar] [CrossRef]
- Scholes, D.T.; Yee, P.Y.; Lindemuth, J.R.; Kang, H.; Onorato, J.; Ghosh, R.; Luscombe, C.K.; Spano, F.C.; Tolbert, S.H.; Schwartz, B.J. The Effects of Crystallinity on Charge Transport and the Structure of Sequentially Processed F4TCNQ-Doped Conjugated Polymer Films. Adv. Funct. Mater. 2017, 27, 1702654. [Google Scholar] [CrossRef]
- Dealy, J.M.; Read, D.J.; Larson, R.G. 6—Tube Models for Linear Polymers—Fundamentals. In Structure and Rheology of Molten Polymers, 2nd ed.; Dealy, J.M., Read, D.J., Larson, R.G., Eds.; Hanser: Munich, Germany, 2018; pp. 197–254. [Google Scholar] [CrossRef]
- Srithep, Y.; Nealey, P.; Turng, L.-S. Effects of annealing time and temperature on the crystallinity and heat resistance behavior of injection-molded poly(lactic acid). Polym Eng Sci 2013, 53, 580–588. [Google Scholar] [CrossRef]
- Mohd Radzuan, N.A.; Sulong, A.B.; Sahari, J. A review of electrical conductivity models for conductive polymer composite. Int. J. Hydrog. Energy 2017, 42, 9262–9273. [Google Scholar] [CrossRef]
- Fan, P.-W.; Chen, W.-L.; Lee, T.-H.; Chiu, Y.-J.; Chen, J.-T. Rayleigh-Instability-Driven Morphology Transformation by Thermally Annealing Electrospun Polymer Fibers on Substrates. Macromolecules 2012, 45, 5816–5822. [Google Scholar] [CrossRef]
- Shalygina, T.A.; Rudenko, M.S.; Nemtsev, I.V.; Parfenov, V.A.; Voronina, S.Y.; Simonov-Emelyanov, I.D.; Borisova, P.E. Influence of the Filler Particles’ Surface Morphology on the Polyurethane Matrix’s Structure Formation in the Composite. Polymers 2021, 13, 3864. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Abhari, R.E.; Mouthuy, P.-A.; Zargar, N.; Brown, C.; Carr, A. Effect of annealing on the mechanical properties and the degradation of electrospun polydioxanone filaments. J. Mech. Behav. Biomed. Mater. 2017, 67, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.W. Novel Theoretical Self-Consistent Mean-Field Approach to Describe the Conductivity of Carbon Fiber-Filled Thermoplastics: Part III—Application of the Concept to Mechanical Properties of Composites and Polymer Solutions. Adv. Eng. Mater. 2020, 22, 2000171. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, B.H.; Kota, A.K.; Gershon, A.L.; Laskowski, C.J.; Kashiwagi, T.; Bruck, H.A.; Raghavan, S.R. Conductivity enhancement of carbon nanotube and nanofiber-based polymer nanocomposites by melt annealing. Polymer 2008, 49, 4846–4851. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Mahmoudifard, M.; Mohamadyar-Toupkanlou, F.; Dodel, M.; Hajarizadeh, A.; Adabi, M.; Soleimani, M. The nanofibrous PAN-PANi scaffold as an efficient substrate for skeletal muscle differentiation using satellite cells. Bioprocess Biosyst. Eng. 2016, 39, 1163–1172. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Wood, M.; Willits, R.K. Short-duration, DC electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics 2006, 27, 328–331. [Google Scholar] [CrossRef]
- Litowczenko, J.; Woźniak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and current achievements in development of multifunctional bioscaffolds for medical application. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lullo, G.A.; Sweeney, S.M.; Körkkö, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the Ligand-binding Sites and Disease-associated Mutations on the Most Abundant Protein in the Human, Type I Collagen*. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solazzo, M.; O’Brien, F.J.; Nicolosi, V.; Monaghan, M.G. The rationale and emergence of electroconductive biomaterial scaffolds in cardiac tissue engineering. APL Bioeng. 2019, 3, 41501. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; van der Werf, K.O.; Koopman, B.F.; Subramaniam, V.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Micromechanical bending of single collagen fibrils using atomic force microscopy. J. Biomed. Mater. Res. 2007, 82A, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherratt, M.J.; Baldock, C.; Louise Haston, J.; Holmes, D.F.; Jones, C.J.; Adrian Shuttleworth, C.; Wess, T.J.; Kielty, C.M. Fibrillin Microfibrils are Stiff Reinforcing Fibres in Compliant Tissues. J. Mol. Biol. 2003, 332, 183–193. [Google Scholar] [CrossRef]
- Akhtar, R.; Schwarzer, N.; Sherratt, M.J.; Watson, R.E.B.; Graham, H.K.; Trafford, A.W.; Mummery, P.M.; Derby, B. Nanoindentation of histological specimens: Mapping the elastic properties of soft tissues. J. Mater. Res. 2009, 24, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Gundiah, N.; B Ratcliffe, M.; A Pruitt, L. Determination of strain energy function for arterial elastin: Experiments using histology and mechanical tests. J. Biomech. 2007, 40, 586–594. [Google Scholar] [CrossRef]
- Laurent, S.; Girerd, X.; Mourad, J.-J.; Lacolley, P.; Beck, L.; Boutouyrie, P.; Mignot, J.-P.; Safar, M. Elastic modulus of the radial artery wall material is not increased in patients with essential hypertension. Arterioscler. Thromb. Vasc. Biol. 1994, 14, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozna, E.R.; Marble, A.E.; Shaw, A.J.; Winter, D.A. Mechanical properties of the ascending thoracic aorta of man1. Cardiovasc Res 1973, 7, 261–265. [Google Scholar] [CrossRef]
- Mawad, D.; Mansfield, C.; Lauto, A.; Perbellini, F.; Nelson, G.W.; Tonkin, J.; Bello, S.O.; Carrad, D.J.; Micolich, A.P.; Mahat, M.M.; et al. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2016, 2, e1601007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Ebrahimi-Barough, S.; Azami, M.; Vahdat, S.; Baharvand, H. Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. J. Biomed. Mater. Res. 2015, 103, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Guarino, V.; Schiavo, L.; Alvarez-Perez, M.A.; Ambrosio, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Med. 2011, 22, 1053–1062. [Google Scholar] [CrossRef]
- McKeon, K.D.; Lewis, A.; Freeman, J.W. Electrospun poly(D,L-lactide) and polyaniline scaffold characterization. J. Appl. Polym. Sci. 2010, 115, 1566–1572. [Google Scholar] [CrossRef]
- Munawar, M.A.; Schubert, D.W. Modeling and optimization of diameter of highly oriented electrospun nanofibers of biopolymer. Unpublished.
- DIN 51562-1:1999-01. Viskosimetrie_-Messung der kinematischen Viskosität mit dem Ubbelohde-Viskosimeter_-Teil_1: Bauform und Durchführung der Messung; Beuth Verlag GmbH: Berlin, Germany, 1999.
- Tam, D.; Ruan, S.; Gao, P.; Yu, T. 10—High-performance ballistic protection using polymer nanocomposites. In Advances in Military Textiles and Personal Equipment: Woodhead Publishing Series in Textiles; Sparks, E., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 213–237. [Google Scholar] [CrossRef]







| Conductive Substrate | Mechanical Properties | Electrical Properties | Cell Line or Tissue | Biological Response |
|---|---|---|---|---|
| PLA/PANI electrospun membranes [48] | - | Four-probe technique, σ = 21 µS/m | H9c2, rat CMs | Myotube formation from H9c2 cells enhanced Cx43 and α-actinin expression improved Ca2+ transients for CMs |
| PCL, PANI electrospun membranes [4] | E = 55.2 MPa | Four-point probe, σ = 63.6 mS/cm | C2C12 | Myotube formation |
| Chitosan, PANI patch [65] | E = 6.73 MPa | Four-probe technique, σ = 0.162 S/cm | Rat MI heart | Improved CV in the infarcted region with healing effects |
| PLCL, PANI electrospun membranes [1] | E = 50 MPa | Four-probe technique, σ = 13.8 mS/cm | Human fibroblasts, NIH-3T3, C2C12 | Improved cell adhesion and metabolic activity |
| PU-AP/PCL porous scaffold [66] | Ec = 4.1 MPa | Four-probe technique, σ = 10−5 S/cm | Neonatal rat CMs | Enhanced Actn4, Cx43, and cTnT2 expressions |
| PANI/PCL patch [67] | - | Two-probe technique, σ = 80 µS/cm | hMSCs | Differentiation of hMSCs to CM-like cells |
| PDLA/PANI electrospun membranes [68] | - | σ = 44 mS/cm | Primary rat muscle cells | Improved cell adhesion and proliferation |
| Gelatin/PANI electrospun membranes [2] | E = 1384 MPa, | Four-probe technique, σ = 17 mS/cm | H9c2 | Smooth muscle-like morphology rich in microfilaments |
| PLGA, PANI electrospun meshes [3] | E = 91.7 MPa | Four-point probe, σ = 3.1 mS/cm | Neonatal rat CMs | Enhanced Cx43 and cTnI expressions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munawar, M.A.; Schubert, D.W. Thermal-Induced Percolation Phenomena and Elasticity of Highly Oriented Electrospun Conductive Nanofibrous Biocomposites for Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 8451. https://doi.org/10.3390/ijms23158451
Munawar MA, Schubert DW. Thermal-Induced Percolation Phenomena and Elasticity of Highly Oriented Electrospun Conductive Nanofibrous Biocomposites for Tissue Engineering. International Journal of Molecular Sciences. 2022; 23(15):8451. https://doi.org/10.3390/ijms23158451
Chicago/Turabian StyleMunawar, Muhammad A., and Dirk W. Schubert. 2022. "Thermal-Induced Percolation Phenomena and Elasticity of Highly Oriented Electrospun Conductive Nanofibrous Biocomposites for Tissue Engineering" International Journal of Molecular Sciences 23, no. 15: 8451. https://doi.org/10.3390/ijms23158451