Fear Extinction and Predictive Trait-Like Inter-Individual Differences in Rats Lacking the Serotonin Transporter
Abstract
:1. Introduction
2. Results
2.1. Body Weight
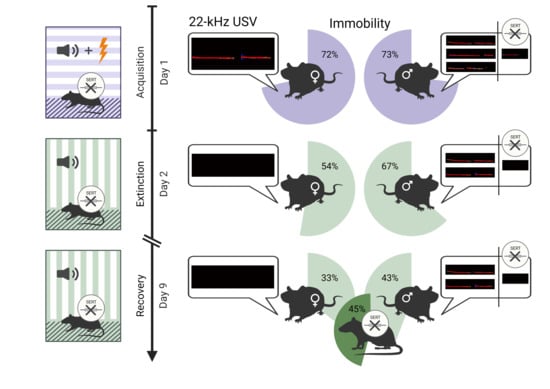
2.2. Differential Fear Conditioning
2.2.1. 22-kHz USV Prevalence
2.2.2. Overall Immobility and 22-kHz USV Total Calling Time
2.2.3. 22-kHz USV: Temporal Emission Pattern
2.2.4. CS+/CS- Presentation: Immobility
2.2.5. CS+/CS- Presentation: 22-kHz USV
2.3. Additional Behavioral Assays
2.3.1. Activity Box
2.3.2. Elevated Plus Maze
2.3.3. Novel Object Recognition
2.3.4. Hot Plate
2.4. Predictors of Inter-Individual Differences in Immobility
3. Discussion
3.1. Fear-Related Behavior
3.1.1. Sex Differences in Fear-Related Behavior
3.1.2. Genotype Differences in Fear-Related Behavior
3.2. Relation of 22-kHz USV and Immobility
3.3. Trait-Like Inter-Individual Differences
3.4. Clinical Implications
4. Conclusions
5. Materials and Methods
5.1. Animals and Housing
5.2. General Procedure
5.3. Activity Box
5.4. Elevated Plus Maze
5.5. Novel Object Recognition
5.6. Differential Fear Conditioning
5.6.1. Setup and Paradigm
5.6.2. Analysis of Immobility
5.6.3. Analysis of Ultrasonic Vocalizations
5.7. Hot Plate
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 9780890425558. [Google Scholar]
- VanElzakker, M.B.; Dahlgren, M.K.; Davis, F.C.; Dubois, S.; Shin, L.M. From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 2014, 113, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervliet, B.; Craske, M.G.; Hermans, D. Fear extinction and relapse: State of the art. Annu. Rev. Clin. Psychol. 2013, 9, 215–248. [Google Scholar] [CrossRef]
- Furini, C.; Myskiw, J.; Izquierdo, I. The learning of fear extinction. Neurosci. Biobehav. Rev. 2014, 47, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Maren, S.; Holmes, A. Stress and fear extinction. Neuropsychopharmacology 2016, 41, 58–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.; Singewald, N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013, 36, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Myers, K.M.; Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 2007, 12, 120–150. [Google Scholar] [CrossRef] [Green Version]
- Lowry, C.A.; Hale, M.W. Serotonin and the neurobiology of anxious states. In Handbook of the Behavioral Neurobiology of Serotonin; Müller, C.P., Jacobs, B.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 379–397. ISBN 9780123746344. [Google Scholar]
- Deakin, J.F.W.; Graeff, F.G. 5-HT and mechanisms of defence. J. Psychopharmacol. 1991, 5, 305–315. [Google Scholar] [CrossRef]
- Bauer, E.P. Serotonin in fear conditioning processes. Behav. Brain Res. 2015, 277, 68–77. [Google Scholar] [CrossRef]
- Guimarães, F.S.; Zangrossi, H., Jr.; Del Ben, C.M.; Cristina, M.; Graeff, F.G. Serotonin in panic and anxiety disorders. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; Volume 21, pp. 667–685. [Google Scholar]
- Gordon, J.A.; Hen, R. The serotonergic system and anxiety. NMM 2004, 5, 27–40. [Google Scholar] [CrossRef]
- Murphy, D.L.; Lesch, K.-P. Targeting the murine serotonin transporter: Insights into human neurobiology. Nat. Rev. Neurosci. 2008, 9, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canli, T.; Lesch, K.-P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.-P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Muller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Garpenstrand, H.; Annas, P.; Ekblom, J.; Oreland, L.; Fredrikson, M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behav. Neurosci. 2001, 115, 358–364. [Google Scholar] [CrossRef]
- Klucken, T.; Alexander, N.; Schweckendiek, J.; Merz, C.J.; Kagerer, S.; Osinsky, R.; Walter, B.; Vaitl, D.; Hennig, J.; Stark, R. Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Soc. Cogn. Affect. Neurosci. 2013, 8, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Hariri, A.R.; Mattay, V.S.; Tessitore, A.; Kolachana, B.; Fera, F.; Goldman, D.; Egan, M.F.; Weinberger, D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002, 297, 400–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gressier, F.; Calati, R.; Balestri, M.; Marsano, A.; Alberti, S.; Antypa, N.; Serretti, A. The 5-HTTLPR polymorphism and posttraumatic stress disorder: A meta-analysis. J. Trauma. Stress 2013, 26, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Li, Q.; Murphy, D.L.; Gold, E.; Crawley, J.N. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: The influence of genetic background. Genes Brain Behav. 2003, 2, 365–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homberg, J.R.; Olivier, J.D.A.; Smits, B.M.G.; Mul, J.D.; Mudde, J.B.; Verheul, M.; Nieuwenhuizen, O.F.M.; Cools, A.R.; Ronken, E.; Cremers, T.; et al. Characterization of the serotonin transporter knockout rat: A selective change in the functioning of the serotonergic system. Neuroscience 2007, 146, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.D.A.; Van Der Hart, M.G.C.; Van Swelm, R.P.L.; Dederen, P.J.; Homberg, J.R.; Cremers, T.; Deen, P.M.T.; Cuppen, E.; Cools, A.R.; Ellenbroek, B.A. A study in male and female 5-HT transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience 2008, 152, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Schipper, P.; Nonkes, L.J.P.; Karel, P.; Kiliaan, A.J.; Homberg, J.R. Serotonin transporter genotype x construction stress interaction in rats. Behav. Brain Res. 2011, 223, 169–175. [Google Scholar] [CrossRef]
- Golebiowska, J.; Hołuj, M.; Potasiewicz, A.; Piotrowska, D.; Kuziak, A.; Popik, P.; Homberg, J.R.; Nikiforuk, A. Serotonin transporter deficiency alters socioemotional ultrasonic communication in rats. Sci. Rep. 2019, 9, 20283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.L.; Molosh, A.I.; Federici, L.M.; Bernabe, C.; Haggerty, D.; Fitz, S.D.; Nalivaiko, E.; Truitt, W.; Shekhar, A. Assessment of fear and anxiety associated behaviors, physiology and neural circuits in rats with reduced serotonin transporter (SERT) levels. Transl. Psychiatry 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Schipper, P.; Kiliaan, A.J.; Homberg, J.R. A mixed polyunsaturated fatty acid diet normalizes hippocampal neurogenesis and reduces anxiety in serotonin transporter knockout rats. Behav. Pharmacol. 2011, 22, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Milad, M.R.; Rauch, S.L.; Pitman, R.K.; Quirk, G.J. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol. Psychol. 2006, 73, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R. Serotonergic modulation of conditioned fear. Scientifica 2012, 2012, 821549. [Google Scholar] [CrossRef] [Green Version]
- Nonkes, L.J.P.; de Pooter, M.; Homberg, J.R. Behavioural therapy based on distraction alleviates impaired fear extinction in male serotonin transporter knockout rats. J. Psychiatry Neurosci. 2012, 37, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luoni, A.; Hulsken, S.; Cazzaniga, G.; Racagni, G.; Homberg, J.R.; Riva, M.A. Behavioural and neuroplastic properties of chronic lurasidone treatment in serotonin transporter knockout rats. Int. J. Neuropsychopharmacol. 2013, 16, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Schipper, P.; Nonkes, L.J.P.; Homberg, J.R. Impaired fear extinction as displayed by serotonin transporter knockout rats housed in open cages is disrupted by IVC cage housing. PLoS ONE 2014, 9, e91472. [Google Scholar] [CrossRef] [Green Version]
- Schipper, P.; Henckens, M.J.A.G.; Lopresto, D.; Kozicz, T.; Homberg, J.R. Acute inescapable stress alleviates fear extinction recall deficits caused by serotonin transporter abolishment. Behav. Brain Res. 2018, 346, 16–20. [Google Scholar] [CrossRef]
- Shan, L.; Guo, H.-Y.; van den Heuvel, C.N.A.M.; van Heerikhuize, J.; Homberg, J.R. Impaired fear extinction in serotonin transporter knockout rats is associated with increased 5-hydroxymethylcytosine in the amygdala. CNS Neurosci. Ther. 2018, 24, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, P.; Brivio, P.; de Leest, D.; Madder, L.; Asrar, B.; Rebuglio, F.; Verheij, M.M.M.; Kozicz, T.; Riva, M.A.; Calabrese, F.; et al. Impaired fear extinction recall in serotonin transporter knockout rats is transiently alleviated during adolescence. Brain Sci. 2019, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Schipper, P.; Hiemstra, M.; Bosch, K.; Nieuwenhuis, D.; Adinolfi, A.; Glotzbach, S.; Borghans, B.; Lopresto, D.; Fernández, G.; Klumpers, F.; et al. The association between serotonin transporter availability and the neural correlates of fear bradycardia. Proc. Natl. Acad. Sci. USA 2019, 116, 25941–25947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Olivier, J.D.A.; Nonkes, L.J.P.; Homberg, J.R. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 2010, 34, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, M.; Uengoer, M.; Schwarting, R.K.W.; Homberg, J.R.; Wöhr, M. Reduced emission of alarm 22-kHz ultrasonic vocalizations during fear conditioning in rats lacking the serotonin transporter. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110072. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Ethotransmission: Communication of emotional states through ultrasonic vocalization in rats. Curr. Opin. Neurobiol. 2013, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Schwarting, R.K.W. Affective communication in rodents: Ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013, 354, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Sales, G.D. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. J. Zool. 1972, 168, 149–164. [Google Scholar] [CrossRef]
- Vivian, J.A.; Miczek, K.A. Morphine attenuates ultrasonic vocalization during agonistic encounters in adult male rats. Psychopharmacology 1993, 111, 367–375. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C.; Agullana, R.; Weiss, S.M. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol. Behav. 1991, 50, 967–972. [Google Scholar] [CrossRef]
- Fendt, M.; Brosch, M.; Wernecke, K.E.A.; Willadsen, M.; Wöhr, M. Predator odour but not TMT induces 22-kHz ultrasonic vocalizations in rats that lead to defensive behaviours in conspecifics upon replay. Sci. Rep. 2018, 8, 11041. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Holland, G. Acoustic characteristics of air puff-induced 22-kHz alarm calls in direct recordings. Neurosci. Biobehav. Rev. 2005, 29, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Mori, Y. The emission of stress-induced 22-kHz calls in female rats is independent of testosterone levels. Horm. Behav. 2015, 69, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Sato, J. Air puff-induced 22-kHz calls in F344 rats. Physiol. Behav. 2016, 155, 237–241. [Google Scholar] [CrossRef]
- Kaltwasser, M.-T. Acoustic startle induced ultrasonic vocalization in the rat: A novel animal model of anxiety? Behav. Brain Res. 1991, 43, 133–137. [Google Scholar] [CrossRef]
- Vivian, J.A.; Farrell, W.J.; Sapperstein, S.B.; Miczek, K.A. Diazepam withdrawal: Effects of diazepam and gepirone on acoustic startle-induced 22 kHz ultrasonic vocalizations. Psychopharmacology 1994, 114, 101–108. [Google Scholar] [CrossRef]
- Cuomo, V.; Cagiano, R.; de Salvia, M.A.; Maselli, M.A.; Renna, G.; Racagni, G. Ultrasonic vocalization in response to unavoidable aversive stimuli in rats: Effects of benzodiazepines. Life Sci. 1988, 43, 485–491. [Google Scholar] [CrossRef]
- Tonoue, T.; Ashida, Y.; Makino, H.; Hata, H. Inhibition of shock-elicited ultrasonic vocalization by opioid peptides in the rat: A psychotropic effect. Psychoneuroendocrinology 1986, 11, 177–184. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Emission of 22 kHz vocalizations in rats as an evolutionary equivalent of human crying: Relationship to depression. Behav. Brain Res. 2019, 363, 1–12. [Google Scholar] [CrossRef]
- Yee, N.; Schwarting, R.K.W.; Fuchs, E.; Wöhr, M. Increased affective ultrasonic communication during fear learning in adult male rats exposed to maternal immune activation. J. Psychiatr. Res. 2012, 46, 1199–1205. [Google Scholar] [CrossRef]
- Borta, A.; Wöhr, M.; Schwarting, R.K.W. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behav. Brain Res. 2006, 166, 271–280. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav. Neurosci. 2008, 122, 310–330. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Schwarting, R.K.W.; Fuchs, E.; Wöhr, M. Juvenile stress potentiates aversive 22-kHz ultrasonic vocalizations and freezing during auditory fear conditioning in adult male rats. Stress 2012, 15, 533–544. [Google Scholar] [CrossRef]
- Lonsdorf, T.B.; Menz, M.M.; Andreatta, M.; Fullana, M.A.; Golkar, A.; Haaker, J.; Heitland, I.; Hermann, A.; Kuhn, M.; Kruse, O.; et al. Don’t fear ’fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci. Biobehav. Rev. 2017, 77, 247–285. [Google Scholar] [CrossRef]
- Wöhr, M.; Borta, A.; Schwarting, R.K.W. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: A dose-response study in the rat. Neurobiol. Learn. Mem. 2005, 84, 228–240. [Google Scholar] [CrossRef]
- Schwarting, R.K.W.; Jegan, N.; Wöhr, M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav. Brain Res. 2007, 182, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Schwarting, R.K.W. Ultrasonic calling during fear conditioning in the rat: No evidence for an audience effect. Anim. Behav. 2008, 76, 749–760. [Google Scholar] [CrossRef]
- De Vry, J.; Benz, U.; Schreiber, R.; Traber, J. Shock-induced ultrasonic vocalization in young adult rats: A model for testing putative anti-anxiety drugs. Eur. J. Pharmacol. 1993, 249, 331–339. [Google Scholar] [CrossRef]
- Graham, L.K.; Yoon, T.; Lee, H.J.; Kim, J.J. Strain and sex differences in fear conditioning: 22 kHz ultrasonic vocalizations and freezing in rats. Psychol. Neurosci. 2009, 2, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Kosten, T.A.; Miserendino, M.J.D.; Bombace, J.C.; Lee, H.J.; Kim, J.J. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav. Brain Res. 2005, 157, 235–244. [Google Scholar] [CrossRef]
- Kosten, T.A.; Lee, H.J.; Kim, J.J. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006, 1087, 142–150. [Google Scholar] [CrossRef]
- Jelen, P.; Soltysik, S.; Zagrodzka, J. 22-kHz Ultrasonic vocalization in rats as an index of anxiety but not fear: Behavioral and pharmacological modulation of affective state. Behav. Brain Res. 2003, 141, 63–72. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Agullana, R.; McGee, L.; Weiss, S.; Blanchard, D.C. Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus). J. Comp. Psychol. 1992, 106, 270–277. [Google Scholar] [CrossRef]
- Shepherd, J.K.; Blanchard, D.C.; Weiss, S.M.; Rodgers, R.J.; Blanchard, R.J. Morphine attenuates antipredator ultrasonic vocalizations in mixed-sex rat colonies. Pharmacol. Biochem. Behav. 1992, 41, 551–558. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, E.S.; Covey, E.; Kim, J.J. Social transmission of fear in rats: The role of 22-kHz ultrasonic distress vocalization. PLoS ONE 2010, 5, e15077. [Google Scholar] [CrossRef] [PubMed]
- Fendt, M.; Gonzalez-Guerrero, C.P.; Kahl, E. Observational fear learning in rats: Role of trait anxiety and ultrasonic vocalization. Brain Sci. 2021, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Willadsen, M.; Kisko, T.M.; Schwarting, R.K.W.; Fendt, M. Sex-dependent effects of Cacna1c haploinsufficiency on behavioral inhibition evoked by conspecific alarm signals in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109849. [Google Scholar] [CrossRef]
- Huang, A.C.W.; Shyu, B.-C.; Hsiao, S.; Chen, T.-C.; He, A.B.-H. Neural substrates of fear conditioning, extinction, and spontaneous recovery in passive avoidance learning: A c-fos study in rats. Behav. Brain Res. 2013, 237, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Furtak, S.C.; Brown, T.H. Limbic-system involvement in rat ultrasonic communications. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–108. ISBN 1569-7339. [Google Scholar]
- McCue, M.G.; LeDoux, J.E.; Cain, C.K. Medial amygdala lesions selectively block aversive pavlovian-instrumental transfer in rats. Front. Behav. Neurosci. 2014, 8, 329. [Google Scholar] [CrossRef] [Green Version]
- Hamdani, S.; White, N.M. Ultrasonic vocalization ratios reflect the influence of motivational state and amygdala lesions on different types of taste avoidance learning. Behav. Brain Res. 2011, 217, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Brown, T.H. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J. Neurosci. 2003, 23, 8713–8721. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Han, J.-S.; Kim, J.J. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J. Neurosci. 2004, 24, 7654–7662. [Google Scholar] [CrossRef] [Green Version]
- Snoeren, E.; Chan, J.; Bovens, A.; Cuppen, E.; Waldinger, M.; Olivier, B.; Oosting, R. Serotonin transporter null mutation and sexual behavior in female rats: 5-HT1A receptor desensitization. J. Sex. Med. 2010, 7, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.D.A.; Cools, A.R.; Olivier, B.; Homberg, J.R.; Cuppen, E.; Ellenbroek, B.A. Stress-induced hyperthermia and basal body temperature are mediated by different 5-HT(1A) receptor populations: A study in SERT knockout rats. Eur. J. Pharmacol. 2008, 590, 190–197. [Google Scholar] [CrossRef] [PubMed]
- de Homberg, J.R.; Boer, S.F.; Raasø, H.; Olivier, J.D.A.; Verheul, M.; Ronken, E.; Cools, A.R.; Ellenbroek, B.A.; Schoffelmeer, A.N.M.; Vanderschuren, L.J.M.J.; et al. Adaptations in pre- and postsynaptic 5-HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology 2008, 200, 367–380. [Google Scholar] [CrossRef] [Green Version]
- El-Ayache, N.; Galligan, J.J. 5-HT3 receptor signaling in serotonin transporter-knockout rats: A female sex-specific animal model of visceral hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G132–G143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, C.; Popa, D.; Fabre, V.; Bouali, S.; Venault, P.; Lesch, K.-P.; Hamon, M.; Adrien, J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J. Neurosci. 2006, 26, 5554–5564. [Google Scholar] [CrossRef] [PubMed]
- Fabre, V.; Beaufour, C.; Evrard, A.; Rioux, A.; Hanoun, N.; Lesch, K.-P.; Murphy, D.L.; Lanfumey, L.; Hamon, M.; Martres, M.-P. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur. J. Neurosci. 2000, 12, 2299–2310. [Google Scholar] [CrossRef]
- Qu, Y.; Villacreses, N.; Murphy, D.L.; Rapoport, S.I. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology 2005, 180, 12–20. [Google Scholar] [CrossRef]
- Li, Q.; Wichems, C.H.; Ma, L.; van de Kar, L.D.; Garcia, F.; Murphy, D.L. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J. Neurochem. 2003, 84, 1256–1265. [Google Scholar] [CrossRef]
- Carrive, P. Dual activation of cardiac sympathetic and parasympathetic components during conditioned fear to context in the rat. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1251–1254. [Google Scholar] [CrossRef]
- Pilz, P.K.; Oedekoven, C. Frequency of the 22 kHz call of rats is modulated by the rhythm of the heart rate. Physiol. Behav. 1995, 57, 325–330. [Google Scholar] [CrossRef]
- Olivier, J.D.A.; Jans, L.A.W.; Blokland, A.; Broers, N.J.; Homberg, J.R.; Ellenbroek, B.A.; Cools, A.R. Serotonin transporter deficiency in rats contributes to impaired object memory. Genes Brain Behav. 2009, 8, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, Y.; Dirven, B.; Janssen, S.; Kröhnke, M.; Barte, R.M.; Middelman, A.; van Bokhoven, H.; Zhou, H.; Homberg, J.R. Perinatal reduction of functional serotonin transporters results in developmental delay. Neuropharmacology 2016, 109, 96–111. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Kasahara, Y.; Hall, F.S.; Lesch, K.-P.; Murphy, D.L.; Uhl, G.R.; Sora, I. Developmental alterations in anxiety and cognitive behavior in serotonin transporter mutant mice. Psychopharmacology 2014, 231, 4119–4133. [Google Scholar] [CrossRef]
- Sartori, S.B.; Hauschild, M.; Bunck, M.; Gaburro, S.; Landgraf, R.; Singewald, N. Enhanced fear expression in a psychopathological mouse model of trait anxiety: Pharmacological interventions. PLoS ONE 2011, 6, e16849. [Google Scholar] [CrossRef] [Green Version]
- Muigg, P.; Hetzenauer, A.; Hauer, G.; Hauschild, M.; Gaburro, S.; Frank, E.; Landgraf, R.; Singewald, N. Impaired extinction of learned fear in rats selectively bred for high anxiety--evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur. J. Neurosci. 2008, 28, 2299–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, G.; Graham, B.M.; Richardson, R. Individual differences in fear relapse. Behav. Res. Ther. 2018, 100, 37–43. [Google Scholar] [CrossRef]
- Ilse, A.; Prameswari, V.; Kahl, E.; Fendt, M. The role of trait anxiety in associative learning during and after an aversive event. Learn. Mem. 2019, 26, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Kreutzmann, J.C.; Marin, M.-F.; Fendt, M.; Milad, M.R.; Ressler, K.; Jovanovic, T. Unconditioned response to an aversive stimulus as predictor of response to conditioned fear and safety: A cross-species study. Behav. Brain Res. 2021, 402, 113105. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Eichendorff, J.; Schwarting, R.K.W. Individual response profiles of male Wistar rats in animal models for anxiety and depression. Behav. Brain Res. 2002, 136, 1–12. [Google Scholar] [CrossRef]
- Pawlak, C.R.; Ho, Y.-J.; Schwarting, R.K.W. Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci. Biobehav. Rev. 2008, 32, 1544–1568. [Google Scholar] [CrossRef]
- Inagaki, H.; Kuwahara, M.; Kikusui, T.; Tsubone, H. The influence of social environmental condition on the production of stress-induced 22 kHz calls in adult male Wistar rats. Physiol. Behav. 2005, 84, 17–22. [Google Scholar] [CrossRef]
- Jones, C.E.; Monfils, M.-H. Dominance status predicts social fear transmission in laboratory rats. Anim. Cogn. 2016, 19, 1051–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, R.A.; Felmingham, K.L.; Falconer, E.M.; Pe Benito, L.; Dobson-Stone, C.; Pierce, K.D.; Schofield, P.R. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol. Psychiatry 2010, 67, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Wannemueller, A.; Moser, D.; Kumsta, R.; Jöhren, H.-P.; Adolph, D.; Margraf, J. Mechanisms, genes and treatment: Experimental fear conditioning, the serotonin transporter gene, and the outcome of a highly standardized exposure-based fear treatment. Behav. Res. Ther. 2018, 107, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Furmark, T.; Tillfors, M.; Garpenstrand, H.; Marteinsdottir, I.; Långström, B.; Oreland, L.; Fredrikson, M. Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci. Lett. 2004, 362, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Smits, B.M.G.; Mudde, J.B.; van de Belt, J.; Verheul, M.; Olivier, J.D.A.; Homberg, J.R.; Guryev, V.; Cools, A.R.; Ellenbroek, B.A.; Plasterk, R.H.A.; et al. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet. Genom. 2006, 16, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.; Wöhr, M.; Seffer, D.; Rippberger, H.; Sungur, A.Ö.; Dietsche, B.; Stein, F.; Sivalingam, S.; Forstner, A.J.; Witt, S.H.; et al. Advanced paternal age as a risk factor for neurodevelopmental disorders: A translational study. Mol. Autism 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.D.; Kisko, T.M.; Vecchia, D.D.; Andreatini, R.; Schwarting, R.K.W.; Wöhr, M. Sex-specific effects of Cacna1c haploinsufficiency on object recognition, spatial memory, and reversal learning capabilities in rats. Neurobiol. Learn. Mem. 2018, 155, 543–555. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willadsen, M.; Uengoer, M.; Sługocka, A.; Schwarting, R.K.W.; Homberg, J.R.; Wöhr, M. Fear Extinction and Predictive Trait-Like Inter-Individual Differences in Rats Lacking the Serotonin Transporter. Int. J. Mol. Sci. 2021, 22, 7088. https://doi.org/10.3390/ijms22137088
Willadsen M, Uengoer M, Sługocka A, Schwarting RKW, Homberg JR, Wöhr M. Fear Extinction and Predictive Trait-Like Inter-Individual Differences in Rats Lacking the Serotonin Transporter. International Journal of Molecular Sciences. 2021; 22(13):7088. https://doi.org/10.3390/ijms22137088
Chicago/Turabian StyleWilladsen, Maria, Metin Uengoer, Anna Sługocka, Rainer K.W. Schwarting, Judith R. Homberg, and Markus Wöhr. 2021. "Fear Extinction and Predictive Trait-Like Inter-Individual Differences in Rats Lacking the Serotonin Transporter" International Journal of Molecular Sciences 22, no. 13: 7088. https://doi.org/10.3390/ijms22137088