Obeticholic Acid Derivative, T-2054 Suppresses Osteoarthritis via Inhibiting NF-κB-Signaling Pathway
Abstract
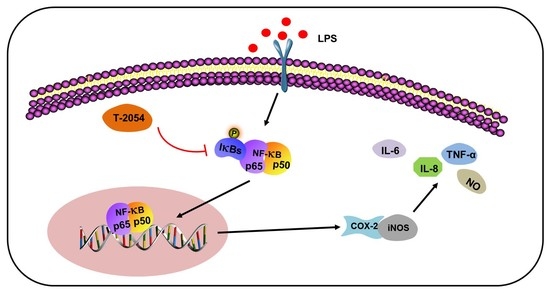
:1. Introduction
2. Results
2.1. Inhibitory Activity on LPS-Induced NO Release
2.2. Effect of T-2054 on Cell Survival in RAW 264.7 Cells and ATDC5 Cells
2.3. T-2054 Inhibited the Expression of IL-6, IL-8 and TNF-α in LPS-Induced RAW 264.7 Cells
2.4. T-2054 Inhibited the Expression of SOX9, MMP9 and ADAMTS5 in TNF-α-Induced ATDC5 Cells
2.5. T-2054 Inhibited LPS-Induced Expression of COX-2 and iNOS in LPS-Induced RAW 264.7 Cells
2.6. T-2054 Inhibited NF-κB Signaling Pathway in LPS-Induced RAW 264.7 Cells and TNF-α-Induced ATDC5 Cells
2.7. T-2054 Reduced Cartilage Damage and the Levels of Cytokines in the Serum in DMM-Induced OA Mouse Model
2.8. T-2054 Had No Effect on Weight and Major Organs Damage in DMM-Induced OA Mouse Model
3. Discussion
4. Materials and Methods
4.1. Identification of Nitric Oxide (NO)
4.2. Cell Viability Assay
4.3. Real-Time Quantitative PCR
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Western Blot
4.6. DMM-Induced OA Mouse Model and Intraperitoneal Administration of the Test Extract
4.7. Histological Analysis
4.8. Detection of Cytokines Levels in Serum
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| OCA | Obeticholic acid |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| TNF-α | Tumor necrosis factor-α |
| ECM | Extracellular matrix |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| MMPs | Matrix metalloproteinases |
| NF-κB | Nuclear factor kappa-B |
| HIF-2α | Hypoxia-inducible factor-2α |
| TCM | Traditional Chinese medicine |
| MTX | Methotrexate |
| FXR | Farnesoid X receptor |
| NO | Nitric oxide |
| ELISA | Enzyme-linked immunosorbent assay |
| LPS | Lipopolysaccharides |
| DMM | Destabilization of the medial meniscus |
References
- Santangelo, K.S.; Nuovo, G.J.; Bertone, A.L. In vivo reduction or blockade of interleukin-1beta in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthr. Cartil. 2012, 20, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Johnson, V.L.; Hunter, D.J. The epidemiology of osteoarthritis. Best. Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthrit Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Fan, Y.Y.; Zhang, C.; Fu, L.; Chen, X.; Xu, D.X. Obeticholic acid differentially regulates hepatic injury and inflammation at different stages of D-galactosamine/lipopolysaccharide-evoked acute liver failure. Eur. J. Pharm. 2019, 850, 150–157. [Google Scholar] [CrossRef]
- Ozawa, M.; Nishida, K.; Yoshida, A.; Saito, T.; Harada, R.; Machida, T.; Ozaki, T. Hyaluronan suppresses mechanical stress-induced expression of catabolic enzymes by human chondrocytes via inhibition of IL-1beta production and subsequent NF-kappaB activation. Inflamm. Res. 2015, 64, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Li, Y.; Li, J.; Feng, S.; Li, S.; Mao, J.; Xie, Y.; Liu, X.; Dong, H.; Zheng, W.; et al. Bufei Yishen Granules Combined with Acupoint Sticking Therapy Suppress Inflammation in Chronic Obstructive Pulmonary Disease Rats: Via JNK/p38 Signaling Pathway. Evid. Based Complement. Altern. 2017, 2017, 1768243. [Google Scholar] [CrossRef] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Chen, C.; Xie, J.; Rajappa, R.; Deng, L.; Fredberg, J.; Yang, L. Interleukin-1beta and tumor necrosis factor-alpha increase stiffness and impair contractile function of articular chondrocytes. Acta Biochim. Biophys Sin. 2015, 47, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.; Wang, W.; Tu, B.; Zhu, Y.; Fan, C.; Li, Y. Overexpression of SOX9 alleviates the progression of human osteoarthritis in vitro and in vivo. Drug Des. Dev. Ther. 2019, 13, 2833–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.S.; Ghosh, S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Chern, C.M.; Zhou, H.; Wang, Y.H.; Chang, C.L.; Chiou, W.F.; Chang, W.T.; Yao, C.H.; Liou, K.T.; Shen, Y.C. Osthole ameliorates cartilage degradation by downregulation of NF-kappaB and HIF-2alpha pathways in an osteoarthritis murine model. Eur. J. Pharm. 2020, 867, 172799. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. Response to Letter to the Editor entitled “Comments on ‘OARSI guidelines for the non-surgical management of knee osteoarthritis’”. Osteoarthr Cartil. 2014, 22, 890–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalunian, K.C. Current advances in therapies for osteoarthritis. Curr. Opin. Rheumatol. 2016, 28, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khera, R.; Allen, A.M.; Murad, M.H.; Loomba, R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology 2015, 62, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Mencarelli, A.; Palladino, G.; Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 2010, 51, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeke, L.; Farre, R.; Verbinnen, B.; Covens, K.; Vanuytsel, T.; Verhaegen, J.; Komuta, M.; Roskams, T.; Chatterjee, S.; Annaert, P.; et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. J. Pathol. 2015, 185, 409–419. [Google Scholar] [CrossRef]
- Pellicciari, R.; Fiorucci, S.; Camaioni, E.; Clerici, C.; Costantino, G.; Maloney, P.R.; Morelli, A.; Parks, D.J.; Willson, T.M. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 2002, 45, 3569–3572. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kao, S.T.; Cheng, C.Y. Acorus tatarinowii Schott extract reduces cerebral edema caused by ischemia-reperfusion injury in rats: Involvement in regulation of astrocytic NKCC1/AQP4 and JNK/iNOS-mediated signaling. BMC Complementary Med. Ther. 2020, 20, 374. [Google Scholar]
- Cavallo, C.; Merli, G.; Borzi, R.M.; Zini, N.; D’Adamo, S.; Guescini, M.; Grigolo, B.; Di Martino, A.; Santi, S.; Filardo, G. Small Extracellular Vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-kappaB dependent inflammatory/catabolic environment of osteoarthritis. Sci. Rep. 2021, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Charmi, G.; Matyjaszewski, K.; Banquy, X.; Pietrasik, J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021, 23, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Agarwal, P.; Grandi, F.; Bruschi, M.; Goodman, S.; Amanatullah, D.; Bhutani, N. Encapsulated Mesenchymal Stromal Cell Microbeads Promote Endogenous Regeneration of Osteoarthritic Cartilage Ex Vivo. Adv. Healthc. Mater. 2021, e2002118. [Google Scholar] [CrossRef]
- Li, B.; Cai, S.; Yang, Y.A.; Chen, S.C.; Chen, R.; Shi, J.B.; Liu, X.H.; Tang, W.J. Novel unsaturated glycyrrhetic acids derivatives: Design, synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 139, 337–348. [Google Scholar] [CrossRef]
- Chen, D.; Lu, D.; Liu, H.; Xue, E.; Zhang, Y.; Shang, P.; Pan, X. Pharmacological blockade of PCAF ameliorates osteoarthritis development via dual inhibition of TNF-alpha-driven inflammation and ER stress. EBioMedicine 2019, 50, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, X.; Zhao, Z.; Chen, J.; Li, C.; Zhao, G. Paeoniflorin inhibited nod-like receptor protein-3 inflammasome and NF-kappaB-mediated inflammatory reactions in diabetic foot ulcer by inhibiting the chemokine receptor CXCR2. Drug Dev. Res. 2020. [Google Scholar] [CrossRef]
- Milenkovic, D.; Deval, C.; Gouranton, E.; Landrier, J.F.; Scalbert, A.; Morand, C.; Mazur, A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS ONE 2012, 7, e29837. [Google Scholar] [CrossRef]
- da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Juni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, e21–e33. [Google Scholar] [CrossRef]
- Sun, M.M.; Beier, F.; Pest, M.A. Recent developments in emerging therapeutic targets of osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Xu, L.; He, Y.; Xu, K.; Chen, Z.; Moqbel, S.A.A.; Ma, C.; Jiang, L.; Ran, J.; Wu, L.; et al. DUSP5 suppresses interleukin-1beta-induced chondrocyte inflammation and ameliorates osteoarthritis in rats. Aging 2020, 12, 26029–26046. [Google Scholar] [CrossRef]
- Wang, K.; Xu, J.; Hunter, D.J.; Ding, C. Investigational drugs for the treatment of osteoarthritis. Expert Opin. Investig. Drugs 2015, 24, 1539–1556. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Ha, J.Y.; Kim, K.M.; Jung, Y.S.; Jung, J.C.; Oh, S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Y.; Chen, X.; Zhang, P.; Li, H.; Chen, L. Synthesis of new ent-labdane diterpene derivatives from andrographolide and evaluation of their anti-inflammatory activities. Eur. J. Med. Chem. 2019, 162, 70–79. [Google Scholar] [CrossRef]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef]
- Yang, L.; Wurm, T.; Sharma Poudel, B.; Krische, M.J. Enantioselective Total Synthesis of Andrographolide and 14-Hydroxy-Colladonin: Carbonyl Reductive Coupling and trans-Decalin Formation by Hydrogen Transfer. Angew. Chem. 2020, 59, 23169–23173. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl 3), S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.; Chen, J.; Hu, X.; Sun, H.; Wu, S.Y.; Chiang, C.M.; Kemper, B.; Chen, L.F.; Kemper, J.K. BRD4 inhibition and FXR activation, individually beneficial in cholestasis, are antagonistic in combination. JCI Insight 2020, 6, 1. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zhang, D.; Chen, Y.; Jin, F. Puerarin alleviates coronary heart disease via suppressing inflammation in a rat model. Gene 2020, 771, 145354. [Google Scholar] [CrossRef]
- Xu, M.; Shen, Y.; Cen, M.; Zhu, Y.; Cheng, F.; Tang, L.; Zheng, X.; Kim, J.J.; Dai, N.; Hu, W. Modulation of the Gut Microbiota-Farnesoid X Receptor Axis Improves Deoxycholic Acid-induced Intestinal Inflammation in Mice. J. Crohns. Colitis 2021. [Google Scholar] [CrossRef]
- Li, S.; Stockl, S.; Lukas, C.; Gotz, J.; Herrmann, M.; Federlin, M.; Grassel, S. hBMSC-Derived Extracellular Vesicles Attenuate IL-1beta-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-inflammatory Signaling Pathways. Front. Bioeng. Biotechnol. 2020, 8, 603598. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.F.; Dos Santos Stein, C.; Moresco, R.N.; Bobinski, F.; Palandi, J.; Fernandes, P.F.; Folmer, V.; da Silva, M.D. Photobiomodulation and Sida tuberculata combination declines the inflammation’s markers in knee-induced osteoarthritis. Laser Med. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Gilbert, S.J.; Caterson, B.; Sandell, L.J.; Archer, C.W. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine articular cartilage. Osteoarthr. Cartil. 2008, 16, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, P.; Yao, T.; Ma, J.; Chen, Z.; Zhu, J.; Gong, Z.; Shen, S.; Fang, X. Novel role of circRSU1 in the progression of osteoarthritis by adjusting oxidative stress. Theranostics 2021, 11, 1877–1900. [Google Scholar] [CrossRef]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; He, L.; Gao, Y.; Jin, C.; Lin, H.; Zhang, L.; Wang, L.; Zhou, Y.; Yao, J.; Duan, Y.; et al. Obeticholic Acid Derivative, T-2054 Suppresses Osteoarthritis via Inhibiting NF-κB-Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 3807. https://doi.org/10.3390/ijms22083807
Guo D, He L, Gao Y, Jin C, Lin H, Zhang L, Wang L, Zhou Y, Yao J, Duan Y, et al. Obeticholic Acid Derivative, T-2054 Suppresses Osteoarthritis via Inhibiting NF-κB-Signaling Pathway. International Journal of Molecular Sciences. 2021; 22(8):3807. https://doi.org/10.3390/ijms22083807
Chicago/Turabian StyleGuo, Dandan, Liming He, Yaoxin Gao, Chenxu Jin, Haizhen Lin, Li Zhang, Liting Wang, Ying Zhou, Jie Yao, Yixin Duan, and et al. 2021. "Obeticholic Acid Derivative, T-2054 Suppresses Osteoarthritis via Inhibiting NF-κB-Signaling Pathway" International Journal of Molecular Sciences 22, no. 8: 3807. https://doi.org/10.3390/ijms22083807