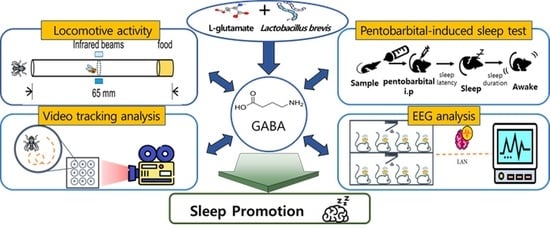
Fermented Gamma Aminobutyric Acid Improves Sleep Behaviors in Fruit Flies and Rodent Models
Abstract
:1. Introduction
2. Results
2.1. Analysis of LB-GABA
2.2. Effects of LB-GABA on Locomotor Activity of Fruit Flies
2.3. Effects of LB-GABA on Locomotor Activity in Caffeine-Induced Awake Model of Fruit Flies
2.4. Effects of LB-GABA on Movement of Fruit Flies
2.5. Effect of LB-GABA on mRNA Expression of Neurotransmitter Receptors in Fruit Flies
2.6. Effects of LB-GABA on Pentobarbital-Induced Sleeping Behavior of Mice
2.7. Effects of LB-GABA on mRNA Expression and Protein Abundance of Neurotransmitter Receptors in Mice
2.8. Effects of LB-GABA on Sleep Pattern of Rats
2.9. Agonist Effects of LB-GABA on Human GABAA Receptor
3. Discussion
4. Materials and Methods
4.1. Preparation and Analysis of LB-GABA
4.2. Fly Maintenance
4.3. Determination of Sleep Behavior
4.4. Rodent Maintenance
4.5. Pentobarbital-Induced Sleep Determination
4.6. EEG Analysis
4.7. mRNA Expression of Neurotransmitter Receptors
4.8. Protein Abundance of Neurotransmitter Receptors
4.9. HEK293 Cell Culture
4.10. Human GABAA (hGABAA) IonFlux HT Agonist Assay
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, H.; Liu, Z.; Xie, F.; Bilal, M.; Liu, L.; Yang, R.; Wang, Z. Microbial production of γ-aminobutyric acid: Applications, state-of-the-art achievements, and future perspectives. Crit. Rev. Biotechnol. 2021, 1–38. [Google Scholar] [CrossRef]
- Sakashita, M.; Nakamura, U.; Horie, N.; Yokoyama, Y.; Kim, M.; Fujita, S. Oral Supplementation Using γ-Aminobutyric Acid and Whey Protein Improves Whole Body Fat-Free Mass in Men After Resistance Training. J. Clin. Med. Res. 2019, 11, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Weir, G.C.; Bonner-Weir, S. GABA Signaling Stimulates β Cell Regeneration in Diabetic Mice. Cell 2017, 168, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Dang, H.; Chen, Z.; Guan, A.; Jin, Y.; Atkinson, M.A.; Kaufman, D.L. γ-Aminobutyric acid regulates both the survival and replication of human β-cells. Diabetes 2013, 62, 3760–3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuypers, K.; Maes, C.; Swinnen, S.P. Aging and GABA. Aging 2018, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Lee, H.S.; Choi, J.; Lee, B.-J.; Park, J.-H.; Park, C.H.; Son, K.H.; Byun, A.K. γ-Aminobutyric acid-salt attenuated high cholesterol/high salt diet induced hypertension in mice. Korean J. Physiol. Pharmacol. 2021, 25, 27–38. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Xia, Y.; Wang, M.; Liao, S.; Li, F.; Yin, J.; Ren, W.-K.; Tan, B.; Yin, Y. Effects of dietary γ-aminobutyric acid supplementation on amino acid profile, intestinal immunity, and microbiota in ETEC-challenged piglets. Food Funct. 2020, 11, 9067–9074. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Quílez, J.; Rafecas, M. γ-Aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of γ-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, R.; Bajpai, V.K.; Baek, K.-H. Production of gaba (γ-Aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwic-Kaminska, K.; Kotysko, M. Sleep quality of student athletes and non-athletes—The role of chronotype, stress and life satisfaction. Sleep. Sci. 2020, 13, 249–255. [Google Scholar] [PubMed]
- Pagan, R. Sleep duration, life satisfaction and disability. Disabil. Health J. 2017, 10, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, F.; Juárez-Aguilar, E.; Santiago-García, J.; Cardinali, D.P. Ghrelin and its interactions with growth hormone, leptin and orexins: Implications for the sleep–wake cycle and metabolism. Sleep Med. Rev. 2014, 18, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, L.; Martin, J.L. Awake at 4 a.m.: Treatment of insomnia with early morning awakenings among older adults. J. Clin. Psychol. 2010, 66, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, D.; Junge, M.F.; Fernando, A.T. Insomnia: Prevalence, consequences and effective treatment. Med. J. Aus. 2013, 199, S36–S40. [Google Scholar] [CrossRef]
- Chen, L.; Bell, J.S.; Visvanathan, R.; Hilmer, S.N.; Emery, T.; Robson, L.; Hughes, J.M.; Tan, E.C.K. The association between benzodiazepine use and sleep quality in residential aged care facilities: A cross-sectional study. BMC Geriatr. 2016, 16, 196. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dong, J.-W.; Zhao, J.-H.; Tang, L.-N.; Zhang, J.-J. Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence. Curr. Neuropharmacol. 2014, 12, 289–302. [Google Scholar] [CrossRef]
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of Diet on Sleep: A Narrative Review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef] [Green Version]
- Nall, A.H.; Shakhmantsir, I.; Cichewicz, K.; Birman, S.; Hirsh, J.; Sehgal, A. Caffeine promotes wakefulness via dopamine signaling in Drosophila. Sci. Rep. 2016, 6, 20938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Jo, K.; Hong, K.-B.; Han, S.H.; Suh, H.J. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamatsu, A.; Yamashita, Y.; Pandharipande, T.; Maru, I.; Kim, M. Effect of oral γ-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Sci. Biotechnol. 2016, 25, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.D.M.; Neto, D.P.D.C.; Júnior, A.I.M.; Prado, F.G.D.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar] [CrossRef]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyazovskiy, V.V.; Delogu, A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neuroscientist 2014, 20, 203–219. [Google Scholar] [CrossRef]
- McKenna, J.T.; Zielinski, M.R.; McCarley, R.W. Neurobiology of REM Sleep, NREM Sleep Homeostasis, and γ Band Oscillations. In Sleep Disorders Medicine; Metzler, J.B., Ed.; AIMS Press: Springfield, MO, USA, 2017; pp. 55–77. [Google Scholar]
- Bon, O.L. Relationships between REM and NREM in the NREM-REM sleep cycle: A review on competing concepts. Sleep Med. 2020, 70, 6–16. [Google Scholar] [CrossRef]
- Gmeiner, F.; Kołodziejczyk, A.; Yoshii, T.; Rieger, D.; Nässel, D.R.; Helfrich-Förster, C. GABA(B) receptors play an essential role in maintaining sleep during the second half of the night in Drosophila melanogaster. J. Exp. Biol. 2013, 216, 3837–3843. [Google Scholar] [CrossRef] [Green Version]
- Karim, N.; Wellendorph, P.; Absalom, N.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. Potency of GABA at human recombinant GABAA receptors expressed in Xenopus oocytes: A mini review. Amino Acids 2013, 44, 1139–1149. [Google Scholar] [CrossRef]
- White, G.; Gurley, D.; Hartnett, C.; Stirling, V.; Gregory, J. Human α and β subunits contribute to the EC50 for GABA at the GABAA receptor expressed in Xenopus oocytes. Recept. Channels 1995, 3, 1–5. [Google Scholar]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, I.; Edden, R.A.; Namgung, E.; Kim, J.; Kim, J. Shorter sleep duration is associated with lower GABA levels in the anterior cingulate cortex. Sleep Med. 2020, 71, 1–7. [Google Scholar] [CrossRef]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [Green Version]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Benitez, S.G.; Jung, S.-R.; Altamirano, L.E.F.; Kruse, M.; Seo, J.-B.; Koh, D.-S.; Muñoz, E.M.; Hille, B. GABAergic signaling in the rat pineal gland. J. Pineal Res. 2016, 61, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Yang, H.; Jeon, Y.-J.; Lee, C.J.; Jin, Y.-H.; Baek, N.-I.; Kim, D.; Kang, S.-M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the edible brown seaweed Ecklonia cava Kjellman induce sleep via positive allosteric modulation of γ-aminobutyric acid type A–benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Ngan, A.; Conduit, R. A Double-blind, Placebo-controlled Investigation of the Effects of Passiflora incarnata (Passionflower) Herbal Tea on Subjective Sleep Quality. Phytother. Res. 2011, 25, 1153–1159. [Google Scholar] [CrossRef]
- Jo, K.; Choi, H.-S.; Jeon, S.; Ahn, C.-W.; Suh, H.J. Nelumbo nucifera Seed Extract Promotes Sleep in Drosophila melanogaster. Biol. Pharm. Bull. 2018, 41, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-S.; Ko, B.S.; Kim, H.D.; Hong, K.-B.; Suh, H.J. Effect of Valerian/Hop Mixture on Sleep-Related Behaviors in Drosophila melanogaster. Biol. Pharm. Bull. 2017, 40, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Jo, K.; Suh, H.J.; Choi, H.-S. Polygonatum sibiricum rhizome promotes sleep by regulating non-rapid eye movement and GABAergic/serotonergic receptors in rodent models. Biomed. Pharmacother. 2018, 105, 167–175. [Google Scholar] [CrossRef]
- Gilestro, G.F. Video tracking and analysis of sleep in Drosophila melanogaster. Nat. Protoc. 2012, 7, 995–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Lee, Y.-C.; Han, K.-S.; Singh, H.; Yoon, M.; Park, J.-H.; Cho, C.-W.; Cho, S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013, 136, 160–163. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, A.-H.; Hwang, J.; Jo, K.; Kim, S.; Ahn, Y.; Suh, H.J.; Choi, H.-S. Fermented Gamma Aminobutyric Acid Improves Sleep Behaviors in Fruit Flies and Rodent Models. Int. J. Mol. Sci. 2021, 22, 3537. https://doi.org/10.3390/ijms22073537
Jeong A-H, Hwang J, Jo K, Kim S, Ahn Y, Suh HJ, Choi H-S. Fermented Gamma Aminobutyric Acid Improves Sleep Behaviors in Fruit Flies and Rodent Models. International Journal of Molecular Sciences. 2021; 22(7):3537. https://doi.org/10.3390/ijms22073537
Chicago/Turabian StyleJeong, A-Hyun, Jisu Hwang, Kyungae Jo, Singeun Kim, Yejin Ahn, Hyung Joo Suh, and Hyeon-Son Choi. 2021. "Fermented Gamma Aminobutyric Acid Improves Sleep Behaviors in Fruit Flies and Rodent Models" International Journal of Molecular Sciences 22, no. 7: 3537. https://doi.org/10.3390/ijms22073537