A ROCK Inhibitor Promotes Graft Survival during Transplantation of iPS-Cell-Derived Retinal Cells
Abstract
:1. Introduction
2. Results
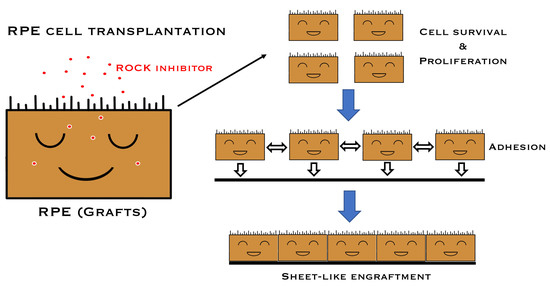
2.1. Promotion of Cell Proliferation and Cell Adhesion in Human iPSC-RPE Cells with Y-27632
2.2. Inhibition of Apoptosis, Promotion of Pigmentation, and Detection of ZO-1, Phospho-Myosin Light Chain 2 in Y-27632-Treated iPS-RPE Cells
2.3. Suppression of Production of Inflammatory Cytokines/Chemokines in Y-27632-Treated iPS-RPE Cells
2.4. Low Immunogenicity in Y-27632-Treated iPS-RPE Cells
2.5. Toxicity of In Vitro iPS-RPE Cells and In Vivo Retinas in a Cynomolgus Monkey by Y-27632 Treatment
2.6. Transplantation of Allogeneic iPS-RPE Cells Together with Y-27632 in an In Vivo Animal Model (a Cynomolgus Monkey)
2.7. Transplantation of Allogeneic iPS-RPE Cells with Y-27632 in MHC Haplotype-Matched or Steroid-Administrated MHC Haplotype-Mismatched Cynomolgus Monkeys
3. Discussion
4. Experimental Procedures
4.1. RPE Cell Culture and Reagents
4.2. Immunohistochemistry and Microscopy
4.3. Quantitative RT-PCR and ELISA
4.4. Flow Cytometry
4.5. Lymphocytes–Grafts Immune Reaction (LGIR) Test
4.6. Transplantation in In Vivo Monkey Models
4.7. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Ohgushi, M.; Matsumura, M.; Eiraku, M.; Murakami, K.; Aramaki, T.; Nishiyama, A.; Muguruma, K.; Nakano, T.; Suga, H.; Ueno, M.; et al. Molecular Pathway and Cell State Responsible for Dissociation-Induced Apoptosis in Human Pluripotent Stem Cells. Cell Stem Cell 2010, 7, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Croze, R.H.; Buchholz, D.E.; Radeke, M.J.; Thi, W.J.; Hu, Q.; Coffey, P.J.; Clegg, D.O. ROCK Inhibition Extends Passage of Pluripotent Stem Cell-Derived Retinal Pigmented Epithelium. STEM CELLS Transl. Med. 2014, 3, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Croze, R.H.; Thi, W.J.; Clegg, D.O. ROCK Inhibition Promotes Attachment, Proliferation, and Wound Closure in Human Embryonic Stem Cell–Derived Retinal Pigmented Epithelium. Transl. Vis. Sci. Technol. 2016, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Garnock-Jones, K.P. Ripasudil: First Global Approval. Drugs 2014, 74, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on Primate Corneal Endothelial Cell Survival In Vitro by a ROCK Inhibitor. Investig. Opthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Zheng, Y.; Bando, H.; Ikuno, Y.; Oshima, Y.; Sawa, M.; Ohji, M.; Tano, Y. Involvement of rho-kinase pathway in contractile activity of rabbit RPE cells in vivo and in vitro. Investig. Opthalmol. Vis. Sci. 2004, 45, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, K.; Takemoto, Y.; Ito, A.; Onda, M.; Morimoto, N.; Mandai, M.; Takahashi, M.; Kato, R.; Osakada, F. Reproducible production and image-based quality evaluation of retinal pigment epithelium sheets from human induced pluripotent stem cells. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Sakamoto, Y.; Okumura, N.; Okahara, N.; Tsuchiya, H.; Torii, R.; Cooper, L.J.; Ban, Y.; Tanioka, H.; Kinoshita, S. Cultivated Corneal Endothelial Cell Sheet Transplantation in a Primate Model. Investig. Opthalmol. Vis. Sci. 2007, 48, 4519–4526. [Google Scholar] [CrossRef]
- Koyanagi, M.; Takahashi, J.; Arakawa, Y.; Doi, D.; Fukuda, H.; Hayashi, H.; Narumiya, S.; Hashimoto, N. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J. Neurosci. Res. 2007, 86, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Braam, S.R.; Nauw, R.; Oostwaard, D.W.-V.; Mummery, C.; Passier, R. Inhibition of ROCK improves survival of human embryonic stem cell-derived cardiomyocytes after dissociation. Ann. N. Y. Acad. Sci. 2010, 1188, 52–57. [Google Scholar] [CrossRef]
- Cechin, S.R.; Dunkley, P.R.; Rodnight, R. Signal Transduction Mechanisms Involved in the Proliferation of C6 Glioma Cells Induced by Lysophosphatidic Acid. Neurochem. Res. 2005, 30, 603–611. [Google Scholar] [CrossRef]
- Moore, M.; Marroquin, B.A.; Gugliotta, W.; Tse, R.; White, S.R. Rho Kinase Inhibition Initiates Apoptosis in Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2004, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.-I.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Miki, A.; Kiryu, J. ROCK Inhibitor-Induced Promotion of Retinal Pigment Epithelial Cell Motility during Wound Healing. J. Ophthalmol. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Maruyama, K.; Himori, N.; Omodaka, K.; Yokoyama, Y.; Shiga, Y.; Morin, R.; Nakazawa, T. The Novel Rho Kinase (ROCK) Inhibitor K-115: A New Candidate Drug for Neuroprotective Treatment in Glaucoma. Investig. Opthalmol. Vis. Sci. 2014, 55, 7126–7136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Nakao, S.; Arita, R.; Kaizu, Y.; Arima, M.; Zhou, Y.; Kita, T.; Yoshida, S.; Kimura, K.; Isobe, T.; et al. Vascular Normalization by ROCK Inhibitor: Therapeutic Potential of Ripasudil (K-115) Eye Drop in Retinal Angiogenesis and Hypoxia. Investig. Opthalmol. Vis. Sci. 2016, 57, 2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nork, T.M.; Murphy, C.J.; Kim, C.B.Y.; Hoeve, J.N.V.; Rasmussen, C.A.; Miller, P.E.; Wabers, H.D.; Neider, M.W.; Dubielzig, R.R.; McCulloh, R.J.; et al. Functional and Anatomic Consequences of Subretinal Dosing in the Cynomolgus Macaque. Arch. Ophthalmol. 2012, 130, 65–75. [Google Scholar] [CrossRef]
- Tharaux, P.-L.; Bukoski, R.C.; Rocha, P.N.; Crowley, S.D.; Ruiz, P.; Nataraj, C.; Howell, D.N.; Kaibuchi, K.; Spurney, R.F.; Coffman, T.M. Rho Kinase Promotes Alloimmune Responses by Regulating the Proliferation and Structure of T Cells. J. Immunol. 2003, 171, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Flynn, R.; Paz, K.; Du, J.; Reichenbach, D.K.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Vulic, A.; Luznik, L.; Macdonald, K.K.P.; Hill, G.R.; et al. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood 2016, 127, 2144–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, S.; Zhan, C.; Lu, J.; Korngold, R.; Schwartz, D.H. Treatment with a Rho Kinase Inhibitor Improves Survival from Graft-Versus-Host Disease in Mice after MHC-Haploidentical Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1104–1111. [Google Scholar] [CrossRef] [Green Version]
- Fujii, S.; Sugita, S.; Futatsugi, Y.; Ishida, M.; Edo, A.; Makabe, K.; Kamao, H.; Iwasaki, Y.; Sakaguchi, H.; Hirami, Y.; et al. A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models. Int. J. Mol. Sci. 2020, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kimura, T.; Futagami, T.; Suegami, S.; Takahashi, M. Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors. Stem Cell Rep. 2016, 7, 619–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, S.; Kamao, H.; Iwasaki, Y.; Okamoto, S.; Hashiguchi, T.; Iseki, K.; Hayashi, N.; Mandai, M.; Takahashi, M. Inhibition of T-Cell Activation by Retinal Pigment Epithelial Cells Derived From Induced Pluripotent Stem Cells. Investig. Opthalmol. Vis. Sci. 2015, 56, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Mandai, M.; Kamao, H.; Takahashi, M. Immunological aspects of RPE cell transplantation. Prog. Retin. Eye Res. 2021, 100950. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Futatsugi, Y.; Ishida, M.; Edo, A.; Takahashi, M. Retinal Pigment Epithelial Cells Derived from Induced Pluripotent Stem (iPS) Cells Suppress or Activate T Cells via Costimulatory Signals. Int. J. Mol. Sci. 2020, 21, 6507. [Google Scholar] [CrossRef]
- Sugita, S.; Makabe, K.; Fujii, S.; Iwasaki, Y.; Kamao, H.; Shiina, T.; Ogasawara, K.; Takahashi, M. Detection of Retinal Pigment Epithelium-Specific Antibody in iPSC-Derived Retinal Pigment Epithelium Transplantation Models. Stem Cell Rep. 2017, 9, 1501–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Yamada, Y.; Aarnink, A.; Suzuki, S.; Masuya, A.; Ito, S.; Ido, D.; Yamanaka, H.; Iwatani, C.; Tsuchiya, H.; et al. Discovery of novel MHC-class I alleles and haplotypes in Filipino cynomolgus macaques (Macaca fascicularis) by pyrosequencing and Sanger sequencing. Immunogenetics 2015, 67, 563–578. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, M.; Sugita, S.; Makabe, K.; Fujii, S.; Futatsugi, Y.; Kamao, H.; Yamasaki, S.; Sakai, N.; Maeda, A.; Mandai, M.; et al. A ROCK Inhibitor Promotes Graft Survival during Transplantation of iPS-Cell-Derived Retinal Cells. Int. J. Mol. Sci. 2021, 22, 3237. https://doi.org/10.3390/ijms22063237
Ishida M, Sugita S, Makabe K, Fujii S, Futatsugi Y, Kamao H, Yamasaki S, Sakai N, Maeda A, Mandai M, et al. A ROCK Inhibitor Promotes Graft Survival during Transplantation of iPS-Cell-Derived Retinal Cells. International Journal of Molecular Sciences. 2021; 22(6):3237. https://doi.org/10.3390/ijms22063237
Chicago/Turabian StyleIshida, Masaaki, Sunao Sugita, Kenichi Makabe, Shota Fujii, Yoko Futatsugi, Hiroyuki Kamao, Suguru Yamasaki, Noriko Sakai, Akiko Maeda, Michiko Mandai, and et al. 2021. "A ROCK Inhibitor Promotes Graft Survival during Transplantation of iPS-Cell-Derived Retinal Cells" International Journal of Molecular Sciences 22, no. 6: 3237. https://doi.org/10.3390/ijms22063237