Biologization of Pcl-Mesh Using Platelet Rich Fibrin (Prf) Enhances Its Regenerative Potential In Vitro
Abstract
:1. Introduction
2. Results
2.1. Prf Provides Matrix and Essential Growth Factor Supply for Human Primary Osteoblasts: Characterization of Prf Coating
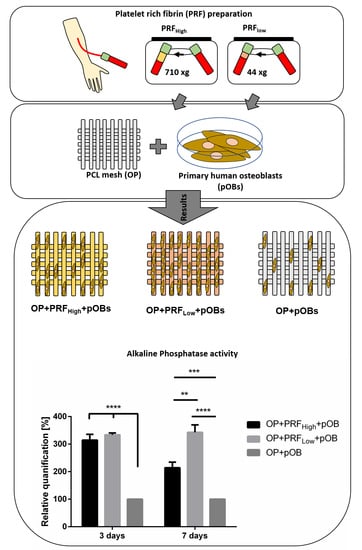
2.2. Prf Coating of OsteoporeTM Meshes Improves Material Biocompatibility: Adhesion and Integration of Primary Osteoblasts
2.3. Stabilization of Growth Factor and Cytokine Content in Cell Culture Supernatants via Prf Coating
3. Discussion
4. Materials and Methods
4.1. OsteoporeTM PCL Mesh
4.2. Platelet Rich Fibrin Assembly and Characterization
4.3. Cell Culture Experiments
4.4. Histochemistry
4.5. Immunofluorescence Staining
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Determination of Alkaline Phosphatase Enzymatic Activity
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghanaati, S.; Al-Maawi, S.; Conrad, T.; Lorenz, J.; Rössler, R.; Sader, R. Biomaterial-based bone regeneration and soft tissue management of the individualized 3D-titanium mesh: An alternative concept to autologous transplantation and flap mobilization. J. Cranio Maxillofac. Surg. 2019. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. In vivo cellular reactions to different biomaterials—Physiological and pathological aspects and their consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential lack of “standardized” processing techniques for production of allogeneic and xenogeneic bone blocks for application in humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Lorenz, J.; Stuebinger, S.; Seitz, O.; Landes, C.; Kovács, A.F.; Kirkpatrick, C.J.; Sader, R.A. Synthetic bone substitute material comparable with xenogeneic material for bone tissue regeneration in oral cancer patients: First and preliminary histological, histomorphometrical and clinical results. Ann. Maxillofac. Surg. 2013, 3, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanaati, S.; Udeabor, S.E.; Barbeck, M.; Willershausen, I.; Kuenzel, O.; Sader, R.A.; Kirkpatrick, C.J. Implantation of silicon dioxide-based nanocrystalline hydroxyapatite and pure phase beta-tricalciumphosphate bone substitute granules in caprine muscle tissue does not induce new bone formation. Head Face Med. 2013, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- De Long, W.G.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. J. Bone Jt. Surg. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Frey, S.P.; Zellweger, R. Bone morphogenetic proteins in clinical applications. ANZ J. Surg. 2007, 77, 626–631. [Google Scholar] [CrossRef]
- Ushirozako, H.; Hasegawa, T.; Ebata, S.; Ohba, T.; Oba, H.; Mukaiyama, K.; Shimizu, S.; Yamato, Y.; Ide, K.; Shibata, Y.; et al. Impact of sufficient contact between the autograft and endplate soon after surgery to prevent nonunion at 12 months following posterior lumbar interbody fusion. J. Neurosurg. Spine 2020, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.D.; Jiang, W.M.; Yang, H.L.; Shi, J.H. Exploratory meta-analysis on dose-related efficacy and complications of rhBMP-2 in anterior cervical discectomy and fusion: 1,539,021 cases from 2003 to 2017 studies. J. Orthop. Transl. 2020, 24, 166–174. [Google Scholar] [CrossRef]
- Gurtner, G.; Werner, S.; Barrandon, Y.; Longaker, M. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Becerra, J.; Visser, R. Insulin-like growth factor-1 (IGF-1) enhances the osteogenic activity of bone morphogenetic protein-6 (BMP-6) in vitro and in vivo, and together have a stronger osteogenic effect than when IGF-1 is combined with BMP-2. J. Biomed. Mater. Res. Part A 2017, 105, 1867–1875. [Google Scholar] [CrossRef]
- Choukroun, V.A.J.; Adda, F.; Schoeffler, C. Uneopportunite en paro-implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Ghanaati, S.; Herrera-Vizcaino, C.; Al-Maawi, S.; Lorenz, J.; Miron, R.J.; Nelson, K.; Schwarz, F.; Choukroun, J.; Sader, R. Fifteen years of platelet rich fibrin (PRF) in dentistry and oromaxillofacial surgery: How high is the level of scientific evidence? J. Oral Implantol. 2018. [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- El Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: A proof of concept of LSCC (low speed centrifugation concept). Eur. J. Trauma Emerg. Surg. 2017. [Google Scholar] [CrossRef] [Green Version]
- Al-Maawi, S.; Herrera-Vizcaino, C.; Dohle, E.; Zrnc, T.A.; Parvini, P.; Schwarz, F.; Sader, R.; Choukroun, J.; Ghanaati, S. Homogeneous pressure influences the growth factor release profiles in solid platelet-rich fibrin matrices and enhances vascular endothelial growth factor release in the solid platelet-rich fibrin plugs. Int. J. Growth Factors Stem Cells Dent. 2018, 1, 8. [Google Scholar] [CrossRef]
- Wend, S.; Kubesch, A.; Orlowska, A.; Al-Maawi, S.; Zender, N.; Dias, A.; Miron, R.J.; Sader, R.; Booms, P.; Kirkpatrick, C.J.; et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J. Mater. Sci. Mater. Med. 2017, 28. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanaati, S.; Mourão, C.; Adam, E.; Sader, R.; Zadeh, H.; Al-Maawi, S. The role of centrifugation process in the preparation of therapeutic blood concentrates: Standardization of the protocols to improve reproducibility. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 41. [Google Scholar] [CrossRef]
- Ghanaati, S.; Al-Maawi, S.; Herrera-Vizcaino, C.; Alves, G.G.; Calasans-Maia, M.D.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Bönig, H.; Mourão, C.F.D.B. A proof of the low speed centrifugation concept in rodents: New perspectives for in vivo research. Tissue Eng. Part C Methods 2018. [Google Scholar] [CrossRef]
- Dohle, E.; el Bagdadi, K.; Sader, R.; Choukroun, J.; Kirkpatrick, C.J.; Ghanaati, S. PRF-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017. [Google Scholar] [CrossRef] [Green Version]
- Al-Maawi, S.; Herrera-Vizcaíno, C.; Orlowska, A.; Willershausen, I.; Sader, R.; Miron, R.J.; Choukroun, J.; Ghanaati, S. Biologization of collagen-based biomaterials using liquid-platelet-rich fibrin: New insights into clinically applicable tissue engineering. Materials 2019, 12, 3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.; Gowda, T.M.; Thomas, R.; Kumar, T.; Mehta, D.S. Biological activation of bone grafts using injectable platelet-rich fibrin. J. Prosthet. Dent. 2019, 121, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Zhang, Y.; Miron, R.J. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet-rich plasma. J. Cosmet. Dermatol. 2019, 18, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Chia-Lai, P.; Orlowska, A.; Al-Maawi, S.; Dias, A.; Zhang, Y.; Wang, X.; Zender, N.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin. Oral Investig. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Bennardo, L.; del Duca, E.; Patruno, C.; Fortunato, L.; Giudice, A.; Nisticò, S.P. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 2020, 33, e13334. [Google Scholar] [CrossRef]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef]
- Young, S.M.; Sundar, G.; Lim, T.C.; Lang, S.S.; Thomas, G.; Amrith, S. Use of bioresorbable implants for orbital fracture reconstruction. Br. J. Ophthalmol. 2017, 101, 1080–1085. [Google Scholar] [CrossRef]
- Kim, D.H.; Yun, W.S.; Shim, J.H.; Park, K.H.; Choi, D.; Park, M.I.; Hwang, S.H.; Kim, S.W. Clinical application of 3-dimensional printing technology for patients with nasal septal deformities: A multicenter study. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 1145–1152. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology-is it still a gold standard. A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Calzado-Martín, A.; Saldaña, L.; Korhonen, H.; Soininen, A.; Kinnari, T.J.; Gómez-Barrena, E.; Tiainen, V.M.; Lappalainen, R.; Munuera, L.; Konttinen, Y.T.; et al. Interactions of human bone cells with diamond-like carbon polymer hybrid coatings. Acta Biomater. 2010, 6, 3325–3338. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Xu, H.H.K. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011, 32, 7503–7513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Kim, J.S.; Lee, J. Improvements of osteoblast adhesion, proliferation, and differentiation in vitro via fibrin network formation in collagen sponge scaffold. J. Biomed. Mater. Res. Part A 2013, 101, 2661–2666. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2017, 1–8. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef] [Green Version]
- Prins, H.J.; Braat, A.K.; Gawlitta, D.; Dhert, W.J.A.; Egan, D.A.; Tijssen-Slump, E.; Yuan, H.; Coffer, P.J.; Rozemuller, H.; Martens, A.C. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem. Cell Res. 2014, 12, 428–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brem, H.; Kodra, A.; Golinko, M.S.; Entero, H.; Stojadinovic, O.; Wang, V.M.; Sheahan, C.M.; Weinberg, A.D.; Woo, S.L.C.; Ehrlich, H.P.; et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J. Investig. Dermatol. 2009, 129, 2275–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahni, A.; Francis, C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000, 96, 3772–3778. [Google Scholar] [CrossRef]
- Dohle, E.; Scherrieble, A.; Doser, M.; Al-Maawi, S.; Hoss, M.; Dauner, M.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Co-culture model for cutaneous wound healing to assess a porous fiber-based drug delivery system. Tissue Eng. Part C Methods. 2020, 26, 475–484. [Google Scholar] [CrossRef]
- Herrera-Vizcaíno, C.; Dohle, E.; Al-Maawi, S.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur. Cell. Mater. 2019, 37, 250–264. [Google Scholar] [CrossRef]
- Dole, N.S.; Mazur, C.M.; Acevedo, C.; Lopez, J.P.; Monteiro, D.A.; Fowler, T.W.; Gludovatz, B.; Walsh, F.; Regan, J.N.; Messina, S.; et al. Osteocyte-intrinsic TGF-β signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017, 21, 2585–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bismar, H.; Klöppinger, T.; Schuster, E.M.; Balbach, S.; Diel, I.; Ziegler, R.; Pfeilschifter, J. Transforming growth factor β (TGF-β) levels in the conditioned media of human bone cells: Relationship to donor age, bone volume, and concentration of TGF-β in human bone matrix in vivo. Bone 1999, 24, 565–569. [Google Scholar] [CrossRef]
- Digiovanni, C.W.; Petricek, J.M. The evolution of rhPDGF-BB in musculoskeletal repair and its role in foot and ankle fusion surgery. Foot Ankle Clin. 2010, 15, 621–640. [Google Scholar] [CrossRef]
- Gao, S.; Lin, R.; Huang, S.; Liang, Y.; Li, X.; Zhang, S.; Ouyang, D.; Li, K.; Zheng, G.; Liao, G. PDGF-BB exhibited therapeutic effects on rat model of bisphosphonate-related osteonecrosis of the jaw by enhancing angiogenesis and osteogenesis. Bone 2019, 144, 115117. [Google Scholar] [CrossRef] [PubMed]
- Gianni-Barrera, R.; Butschkau, A.; Uccelli, A.; Certelli, A.; Valente, P.; Bartolomeo, M.; Groppa, E.; Burger, M.G.; Hlushchuk, R.; Heberer, M.; et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis 2018, 21, 883–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yu, W.; Niibe, K.; Zhang, W.; Egusa, H.; Tang, T.; Jiang, X. The effects of platelet-derived growth factor-BB on bone marrow stromal cell-mediated vascularized bone regeneration. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamano, S.; Haku, K.; Yamanaka, T.; Dai, J.; Takayama, T.; Shohara, R.; Tachi, K.; Ishioka, M.; Hanatani, S.; Karunagaran, S.; et al. The effect of a bioactive collagen membrane releasing PDGF or GDF-5 on bone regeneration. Biomaterials 2014, 35, 2446–2453. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef]
- Cai, L.; Lin, D.; Chai, Y.; Yuan, Y.; Liu, C. MBG scaffolds containing chitosan microspheres for binary delivery of IL-8 and BMP-2 for bone regeneration. J. Mater. Chem. B 2018, 6, 4453. [Google Scholar] [CrossRef]
- Yang, A.; Lu, Y.; Xing, J.; Li, Z.; Yin, X.; Dou, C.; Dong, S.; Luo, F.; Xie, Z.; Hou, T.; et al. IL-8 Enhances therapeutic effects of BMSCs on bone regeneration via CXCR2-mediated PI3k/akt signaling pathway. Cell. Physiol. Biochem. 2018, 48, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chai, Y.; Ma, Y.; Duan, B.; Yuan, Y.; Liu, C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials 2019, 196, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Lee, K.M.; Kim, S.H.; Kim, S.H.; Jung, Y.; Kim, S.H.; Park, K.H.; Choi, Y.; Ryu, H.A.; Choi, W.J.; et al. Synergistic action of IL-8 and bone marrow concentrate on cartilage regeneration through upregulation of chondrogenic transcription factors. Tissue Eng. Part A 2016, 22, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Spiguel, L.; Shenaq, D.; Zhong, M.; Wietholt, C.; He, T.-C.; Reid, R.R. The role of RANK-RANKL-OPG axis in cranial suture homeostasis. Plast. Reconstr. Surg. 2010, 126, 100. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Kapasa, E.; Giannoudis, P.; Jia, X.; Hatton, P.; Yang, X. The Effect of RANKL/OPG balance on reducing implant complications. J. Funct. Biomater. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Konrad, L.; Gotzen, L.; Printz, H.; Ramaswamy, A.; Hofmann, C. Bioengineered human bone tissue using autogenous osteoblasts cultured on different biomatrices. J. Biomed. Mater. Res. Part A 2003, 67, 191–199. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maawi, S.; Dohle, E.; Lim, J.; Weigl, P.; Teoh, S.H.; Sader, R.; Ghanaati, S. Biologization of Pcl-Mesh Using Platelet Rich Fibrin (Prf) Enhances Its Regenerative Potential In Vitro. Int. J. Mol. Sci. 2021, 22, 2159. https://doi.org/10.3390/ijms22042159
Al-Maawi S, Dohle E, Lim J, Weigl P, Teoh SH, Sader R, Ghanaati S. Biologization of Pcl-Mesh Using Platelet Rich Fibrin (Prf) Enhances Its Regenerative Potential In Vitro. International Journal of Molecular Sciences. 2021; 22(4):2159. https://doi.org/10.3390/ijms22042159
Chicago/Turabian StyleAl-Maawi, Sarah, Eva Dohle, Jing Lim, Paul Weigl, Swee Hin Teoh, Robert Sader, and Shahram Ghanaati. 2021. "Biologization of Pcl-Mesh Using Platelet Rich Fibrin (Prf) Enhances Its Regenerative Potential In Vitro" International Journal of Molecular Sciences 22, no. 4: 2159. https://doi.org/10.3390/ijms22042159