Isoflurane Potentiation of GABAA Receptors Is Reduced but Not Eliminated by the β3(N265M) Mutation
Abstract
:1. Introduction
2. Results
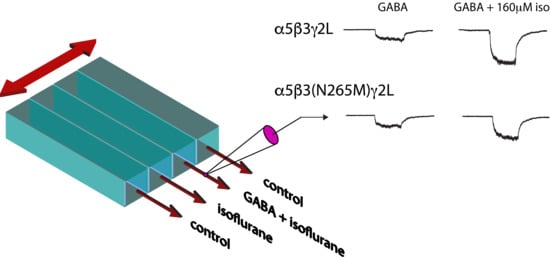
2.1. Isoflurane Modulation of GABA—Elicited Currents
2.2. Direct Activation of GABAA Receptors by Isoflurane
3. Discussion
3.1. Identification of Molecular Targets of Anesthetics
3.2. Contribution of GABAAR Modulation to Memory Suppression
4. Materials and Methods
4.1. Cell Culture and Receptor Expression
4.2. Electrophysiological Recordings
4.3. Solution Preparation and Application
4.4. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antkowiak, B.; Rudolph, U. New insights in the systemic and molecular underpinnings of general anesthetic actions mediated by gamma-aminobutyric acid A receptors. Curr. Opin. Anaesthesiol. 2016, 29, 447–453. [Google Scholar] [CrossRef]
- Antkowiak, B.; Rammes, G. GABA(A) receptor-targeted drug development -New perspectives in perioperative anesthesia. Expert Opin. Drug Discov. 2019, 14, 683–699. [Google Scholar] [CrossRef]
- Hemmings, H.C.; Riegelhaupt, P.M.; Kelz, M.B.; Solt, K.; Eckenhoff, R.G.; Orser, B.A.; Goldstein, P.A. Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharm. Sci. 2019, 40, 464–481. [Google Scholar] [CrossRef]
- Reynolds, D.S.; Rosahl, T.W.; Cirone, J.; O’Meara, G.F.; Haythornthwaite, A.; Newman, R.J.; Myers, J.; Sur, C.; Howell, O.; Rutter, A.R.; et al. Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J. Neurosci. 2003, 23, 8608–8617. [Google Scholar] [CrossRef] [Green Version]
- Jurd, R.; Arras, M.; Lambert, S.; Drexler, B.; Siegwart, R.; Crestani, F.; Zaugg, M.; Vogt, K.E.; Ledermann, B.; Antkowiak, B.; et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003, 17, 250–252. [Google Scholar] [CrossRef]
- Zeller, A.; Arras, M.; Jurd, R.; Rudolph, U. Mapping the contribution of beta3-containing GABAA receptors to volatile and intravenous general anesthetic actions. BMC Pharm. 2007, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Sonner, J.M.; Werner, D.F.; Elsen, F.P.; Xing, Y.; Liao, M.; Harris, R.A.; Harrison, N.L.; Fanselow, M.S.; Eger, E.I., II; Homanics, G.E. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology 2007, 106, 107–113. [Google Scholar] [CrossRef]
- Werner, D.F.; Swihart, A.; Rau, V.; Jia, F.; Borghese, C.M.; McCracken, M.L.; Iyer, S.; Fanselow, M.S.; Oh, I.; Sonner, J.M.; et al. Inhaled anesthetic responses of recombinant receptors and knockin mice harboring alpha2(S270H/L277A) GABA(A) receptor subunits that are resistant to isoflurane. J. Pharm. Exp. 2011, 336, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.; Arras, M.; Vogt, K.E.; Rudolph, U. Isoflurane-induced surgical tolerance mediated only in part by beta3-containing GABA(A) receptors. Eur. J. Pharmacol. 2005, 516, 23–27. [Google Scholar] [CrossRef]
- Liao, M.; Sonner, J.M.; Jurd, R.; Rudolph, U.; Borghese, C.M.; Harris, R.A.; Laster, M.J.; Eger, E.I. Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth. Analg. 2005, 101, 412–418. [Google Scholar] [CrossRef]
- Siegwart, R.; Jurd, R.; Rudolph, U. Molecular determinants for the action of general anesthetics at recombinant α(2)β(3)γ(2) γ-aminobutyric acid(A) receptors. J. Neurochem. 2002, 80, 140–148. [Google Scholar] [CrossRef]
- Krasowski, M.D.; Harrison, N.L. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br. J. Pharm. 2000, 129, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Mihic, S.J.; Ye, Q.; Wick, M.J.; Koltchine, V.V.; Krasowski, M.D.; Finn, S.E.; Mascia, M.P.; Valenzuela, C.F.; Hanson, K.K.; Greenblatt, E.P.; et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 1997, 389, 385–389. [Google Scholar] [CrossRef]
- Zarnowska, E.D.; Rodgers, F.C.; Oh, I.; Rau, V.; Lor, C.; Laha, K.T.; Jurd, R.; Rudolph, U.; Eger, E.I.N.; Pearce, R.A. Etomidate blocks LTP and impairs learning but does not enhance tonic inhibition in mice carrying the N265M point mutation in the beta3 subunit of the GABA(A) receptor. Neuropharmacology 2015, 93, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Sperk, G.; Schwarzer, C.; Tsunashima, K.; Fuchs, K.; Sieghart, W. GABA(A) receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience 1997, 80, 987–1000. [Google Scholar] [CrossRef]
- Cheng, V.Y.; Martin, L.J.; Elliott, E.M.; Kim, J.H.; Mount, H.T.; Taverna, F.A.; Roder, J.C.; Macdonald, J.F.; Bhambri, A.; Collinson, N.; et al. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J. Neurosci. 2006, 26, 3713–3720. [Google Scholar] [CrossRef]
- Saab, B.J.; Maclean, A.J.; Kanisek, M.; Zurek, A.A.; Martin, L.J.; Roder, J.C.; Orser, B.A. Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology 2010, 113, 1061–1071. [Google Scholar] [CrossRef] [Green Version]
- Burkat, P.M.; Lor, C.; Perouansky, M.; Pearce, R.A. Enhancement of α5-Containing γ-Aminobutyric Acid Type A Receptors by the Nonimmobilizer 1,2-Dichlorohexafluorocyclobutane (F6) Is Abolished by the beta3(N265M) Mutation. Anesth. Analg. 2014, 119, 1277–1284. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, K.; Jenkins, A.; Paraskevakis, I.; Harrison, N.L. Volatile anesthetic actions on the GABAA receptors: Contrasting effects of alpha 1(S270) and beta 2(N265) point mutations. Neuropharmacology 2002, 42, 337–345. [Google Scholar] [CrossRef]
- Rudolph, U.; Antkowiak, B. Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci. 2004, 5, 709–720. [Google Scholar] [CrossRef]
- Rudolph, U.; Mohler, H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharm. Toxicol. 2004, 44, 475–498. [Google Scholar] [CrossRef]
- Hörtnagl, H.; Tasan, R.O.; Wieselthaler, A.; Kirchmair, E.; Sieghart, W.; Sperk, G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 2013, 236, 345–372. [Google Scholar] [CrossRef] [Green Version]
- Pirker, S.; Schwarzer, C.; Wieselthaler, A.; Sieghart, W.; Sperk, G. GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 2000, 101, 815–850. [Google Scholar] [CrossRef]
- Delorey, T.M.; Handforth, A.; Anagnostaras, S.G.; Homanics, G.E.; Minassian, B.A.; Asatourian, A.; Fanselow, M.S.; Delgado-Escueta, A.; Ellison, G.D.; Olsen, R.W. Mice lacking the β3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J. Neurosci. 1998, 18, 8505–8514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, S.; Perouansky, M.; Pearce, R.A. Isoflurane enhances both fast and slow synaptic inhibition in the hippocampus at amnestic concentrations. Anesthesiology 2012, 116, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, V.; Oh, I.; Liao, M.; Bodarky, C.; Fanselow, M.S.; Homanics, G.E.; Sonner, J.M.; Eger, E.I., II. Gamma-aminobutyric acid type A receptor beta3 subunit forebrain-specific knockout mice are resistant to the amnestic effect of isoflurane. Anesth. Analg. 2011, 113, 500–504. [Google Scholar]
- Barnard, E.A.; Skolnick, P.; Olsen, R.W.; Mohler, H.; Sieghart, W.; Biggio, G.; Braestrup, C.; Bateson, A.N.; Langer, S.Z. International union of pharmacology-xv-subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998, 50, 291–313. [Google Scholar]
- Olsen, R.W.; Sieghart, W. GABA(A) receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology 2009, 56, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalan, S.P.; Bushman, J.D.; Mercado, J.L.; Sancar, F.; Christopherson, K.R.; Boileau, A.J. Optimized expression vector for ion channel studies in Xenopus oocytes and mammalian cells using alfalfa mosaic virus. Pflug. Arch. Eur. J. Physiol. 2007, 454, 155–163. [Google Scholar] [CrossRef]
- Chesney, M.A.; Perouansky, M.; Pearce, R.A. Differential uptake of volatile agents into brain tissue in vitro. Measurement and application of a diffusion model to determine concentration profiles in brain slices. Anesthesiology 2003, 99, 122–130. [Google Scholar] [CrossRef]
- Zarnowska, E.D.; Pearce, R.A.; Saad, A.A.; Perouansky, M. The gamma-subunit governs the susceptibility of recombinant gamma-aminobutyric acid type A receptors to block by the nonimmobilizer 1,2-dichlorohexafluorocyclobutane (F6, 2N). Anesth. Analg. 2005, 101, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Czajkowski, C.; Pearce, R.A. Rapid and direct modulation of GABAA receptors by halothane. Anesthesiology 2000, 92, 1366–1375. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lor, C.; Perouansky, M.; Pearce, R.A. Isoflurane Potentiation of GABAA Receptors Is Reduced but Not Eliminated by the β3(N265M) Mutation. Int. J. Mol. Sci. 2020, 21, 9534. https://doi.org/10.3390/ijms21249534
Lor C, Perouansky M, Pearce RA. Isoflurane Potentiation of GABAA Receptors Is Reduced but Not Eliminated by the β3(N265M) Mutation. International Journal of Molecular Sciences. 2020; 21(24):9534. https://doi.org/10.3390/ijms21249534
Chicago/Turabian StyleLor, Chong, Misha Perouansky, and Robert A. Pearce. 2020. "Isoflurane Potentiation of GABAA Receptors Is Reduced but Not Eliminated by the β3(N265M) Mutation" International Journal of Molecular Sciences 21, no. 24: 9534. https://doi.org/10.3390/ijms21249534