Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress
Abstract
:1. Introduction
2. Air Pollution Mixtures and Sources
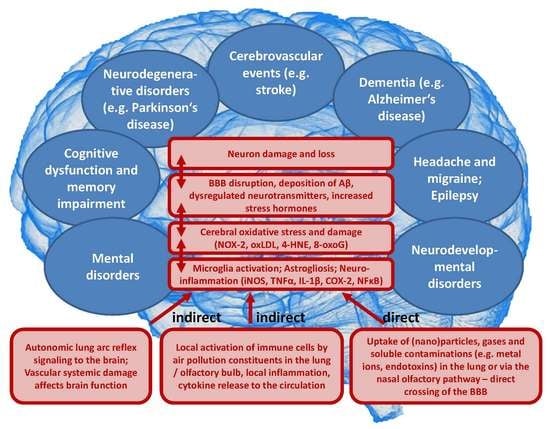
3. Pathophysiology of Air-Pollution-Induced Disorders
4. Evidence from Human and Animal Studies
4.1. Human Observational/Epidemiological Studies
4.1.1. Cerebrovascular Events
4.1.2. Dementia
4.1.3. Parkinson’s Disease
4.1.4. Cognitive Decline
4.1.5. Headache and Migraine
4.1.6. Epilepsy
4.1.7. Neurodevelopmental Disorders
4.1.8. Mental Disorders
4.2. Animal Experimental Studies
5. Neurological Complications of Coronavirus Disease 2019 (COVID-19) and Air Pollution
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3NT | 3-nitrotyrosine |
| 4-HNE | 4-hydroxynonenal |
| 8-oxoG | 8-hydroxyguanosine |
| Aβ | amyloid beta peptide |
| ACE2 | angiotensin-converting enzyme 2 |
| BBB | blood–brain barrier |
| CD68 | cluster of differentiation 68—also macrosialin |
| CI | confidence interval |
| CNS | central nervous system |
| COVID-19 | coronavirus disease 2019 |
| COX-2 | inducible cyclooxygenase (isoform 2) |
| DEP | diesel exhaust particles |
| EC | elemental or black carbon |
| γ-H2AX | H2A histone family member X |
| GBD | Global Burden of Disease |
| GEMM | Global Exposure-Mortality Model |
| gp91phox | catalytic subunit of the phagocytic NADPH oxidase (isoform 2)—also NOX-2 |
| H3K9me2/me3 | histone 3 lysine 9 di- and trimethylation (epigenetic marks) |
| HR | hazard ratio |
| ICAM-1 | intercellular adhesion molecule 1 |
| INF-γ | interferon gamma |
| IHME | Institute for Health Metrics and Evaluation |
| IL-1β | interleukin-1β |
| iNOS | inducible nitric oxide synthase |
| IQR | interquartile range |
| Ly6g | lymphocyte antigen 6 complex locus G6D |
| MCP-1 | monocyte chemoattractant protein-1—also CCL2 |
| MyD88 | myeloid differentiation primary response 88 protein |
| NFκB | nuclear factor ‘kappa-light- chain-enhancer’ of activated B-cells |
| NOx | oxides of nitrogen |
| NOX-2 | catalytic subunit of the phagocytic NADPH oxidase (isoform 2)—also gp91phox |
| OC | organic carbon |
| OR | odds ratio |
| oxLDL | oxidized low density lipoprotein |
| p47phox | regulatory subunit of the phagocytic NADPH oxidase (isoform 2) |
| PAHs | polycyclic aromatic hydrocarbons |
| PCBs | polychlorinated biphenyls |
| PM0.1 | particulate matter < 0.1 µm (ultrafine particles) |
| PM2.5 | particulate matter < 2.5 µm (fine particles) |
| PM10 | particulate matter with a diameter between 2.5 and 10 µm (coarse particles) |
| RANTES | chemokine (C-C motif) ligand 5—also CCL5 |
| ROS | reactive oxygen species |
| RR | relative risk |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SNS | sympathetic nervous system |
| TLR4 | Toll-like receptor 4 |
| TNFα | tumor necrosis factor alpha |
| TNFR2 | tumor necrosis factor receptor type 2 |
| UK | United Kingdom |
| US | United States |
| VOCs | volatile organic compounds |
| WHO | World Health Organization |
References
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Preventing Disease Through Healthy Environments: A Global Assessment of the Burden of Disease From Environmental Risks. Available online: https://apps.who.int/iris/handle/10665/204585 (accessed on 29 April 2020).
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. 9 out of 10 People Worldwide Breathe Polluted Air, but More Countries Are Taking Action. Available online: https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 29 April 2020).
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Poschl, U.; Fnais, M.; Daiber, A.; Munzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart. J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roque, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiong, L.; Tang, M. Toxicity of inhaled particulate matter on the central nervous system: Neuroinflammation, neuropsychological effects and neurodegenerative disease. J. Appl. Toxicol. 2017, 37, 644–667. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Munzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart. J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef] [Green Version]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [Green Version]
- Poschl, U.; Shiraiwa, M. Multiphase chemistry at the atmosphere-biosphere interface influencing climate and public health in the anthropocene. Chem. Rev. 2015, 115, 4440–4475. [Google Scholar] [CrossRef]
- Orru, H.; Ebi, K.L.; Forsberg, B. The Interplay of Climate Change and Air Pollution on Health. Curr Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Air Pollution and Climate Change. Available online: https://ec.europa.eu/environ-ment/integration/research/newsalert/pdf/24si_en.pdf (accessed on 4 May 2020).
- Peden, D.B. Pollutants and asthma: Role of air toxics. Environ. Health Perspect. 2002, 110, 565–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarze, P.E.; Ovrevik, J.; Hetland, R.B.; Becher, R.; Cassee, F.R.; Lag, M.; Lovik, M.; Dybing, E.; Refsnes, M. Importance of size and composition of particles for effects on cells in vitro. Inhal. Toxicol. 2007, 19, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef]
- Munzel, T.; Steven, S.; Frenis, K.; Hahad, O.; Daiber, A. Environmental factors such as noise and air pollution and vascular disease. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report on the Global Tobacco Epidemic, 2017 Monitoring Tobacco Use and Prevention Policies. Available online: https://apps.who.int/iris/bitstream/handle/10665/255874/9789241512824-eng.pdf?sequence=1 (accessed on 4 May 2020).
- Lelieveld, J.; Pozzer, A.; Poschl, U.; Fnais, M.; Haines, A.; Munzel, T. Loss of life expectancy from air pollution compared to other risk factors: A worldwide perspective. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Munzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, G.; Danti, S.; Carlesi, C.; Borin, G. Danger in the Air: Air Pollution and Cognitive Dysfunction. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 333–341. [Google Scholar] [CrossRef]
- Wilson, S.J.; Miller, M.R.; Newby, D.E. Effects of Diesel Exhaust on Cardiovascular Function and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 819–836. [Google Scholar] [CrossRef]
- Moulton, P.V.; Yang, W. Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public Health 2012, 2012, 472751. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garciduenas, L.; Solt, A.C.; Henriquez-Roldan, C.; Torres-Jardon, R.; Nuse, B.; Herritt, L.; Villarreal-Calderon, R.; Osnaya, N.; Stone, I.; Garcia, R.; et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Heidari Nejad, S.; Takechi, R.; Mullins, B.J.; Giles, C.; Larcombe, A.N.; Bertolatti, D.; Rumchev, K.; Dhaliwal, S.; Mamo, J. The effect of diesel exhaust exposure on blood-brain barrier integrity and function in a murine model. J. Appl. Toxicol. 2015, 35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, S.; Farbood, Y.; Gharib-Naseri, M.K.; Goudarzi, G.; Rashno, M.; Maleki, H.; Bakhtiari, N.; Nesari, A.; Khoshnam, S.E.; Dianat, M.; et al. Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci. 2020, 242, 117210. [Google Scholar] [CrossRef]
- Robinson, R.K.; Birrell, M.A.; Adcock, J.J.; Wortley, M.A.; Dubuis, E.D.; Chen, S.; McGilvery, C.M.; Hu, S.; Shaffer, M.S.P.; Bonvini, S.J.; et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J. Allergy Clin. Immunol. 2018, 141, 1074–1084 e1079. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.M.; Hazari, M.S.; Farraj, A.K. Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc. Toxicol. 2015, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Diez Roux, A.V.; Castro-Diehl, C.; Cosselman, K.; Golden, S.H.; Hazlehurst, M.F.; Szpiro, A.; Vedal, S.; Kaufman, J.D. The Association between Long-Term Air Pollution and Urinary Catecholamines: Evidence from the Multi-Ethnic Study of Atherosclerosis. Environ. Health Perspect. 2019, 127, 57007. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef]
- D’Angiulli, A. Severe Urban Outdoor Air Pollution and Children’s Structural and Functional Brain Development, From Evidence to Precautionary Strategic Action. Front. Public Health 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Nakagawa, S. Planetary Health and the Future of Human Capacity: The Increasing Impact of Planetary Distress on the Human Brain. Challenges 2018, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Block, M.L.; Calderon-Garciduenas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Kroller-Schon, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Vujacic-Mirski, K.; Kuntic, M.; Bayo Jimenez, M.T.; Helmstadter, J.; Steven, S.; et al. Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction-Signatures of the internal exposome. Biofactors 2019, 45, 495–506. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S. Air pollution and cardiovascular events. N. Engl. J. Med. 2007, 356, 2104–2105. [Google Scholar] [CrossRef]
- Mateen, F.J.; Brook, R.D. Air pollution as an emerging global risk factor for stroke. JAMA 2011, 305, 1240–1241. [Google Scholar] [CrossRef]
- Shah, A.S.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Wang, X.; Deng, Q.; Fan, W.Y.; Wang, W.Y. An evidence-based appraisal of global association between air pollution and risk of stroke. Int. J. Cardiol. 2014, 175, 307–313. [Google Scholar] [CrossRef]
- Scheers, H.; Jacobs, L.; Casas, L.; Nemery, B.; Nawrot, T.S. Long-Term Exposure to Particulate Matter Air Pollution Is a Risk Factor for Stroke: Meta-Analytical Evidence. Stroke 2015, 46, 3058–3066. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Eliot, M.N.; Wellenius, G.A. Short-term changes in ambient particulate matter and risk of stroke: A systematic review and meta-analysis. J. Am. Heart. Assoc. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Wang, J.; Jiang, Q.; He, Z.; Huang, Y.; Li, Z.; Cai, L.; Cao, S. Long-term exposure to PM2.5 and stroke: A systematic review and meta-analysis of cohort studies. Environ. Res. 2019, 177, 108587. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Shi, Y.; Chen, N.; Wang, H.; Chen, T. Ambient fine particulate matter and hospital admissions for ischemic and hemorrhagic strokes and transient ischemic attack in 248 Chinese cities. Sci. Total Environ. 2020, 715, 136896. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, F.; Yang, X.; Liu, F.; Li, J.; Xiao, Q.; Chen, J.; Liu, X.; Cao, J.; Shen, C.; et al. Long term exposure to ambient fine particulate matter and incidence of stroke: Prospective cohort study from the China-PAR project. BMJ 2019, 367, l6720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nhung, N.T.T.; Schindler, C.; Chau, N.Q.; Hanh, P.T.; Hoang, L.T.; Dien, T.M.; Thanh, N.T.N.; Kunzli, N. Exposure to air pollution and risk of hospitalization for cardiovascular diseases amongst Vietnamese adults: Case-crossover study. Sci. Total Environ. 2020, 703, 134637. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Sharma, M. Cause and Age-specific premature mortality attributable to PM2.5 Exposure: An analysis for Million-Plus Indian cities. Sci. Total Environ. 2020, 710, 135230. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.L.S.; Andersson, N.; Stockfelt, L.; Andersson, E.M.; Nilsson Sommar, J.; Eneroth, K.; Gidhagen, L.; Johansson, C.; Lager, A.; Leander, K.; et al. Long-Term Exposure to Particulate Air Pollution, Black Carbon, and Their Source Components in Relation to Ischemic Heart Disease and Stroke. Environ. Health Perspect. 2019, 127, 107012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivanco-Hidalgo, R.M.; Avellaneda-Gomez, C.; Dadvand, P.; Cirach, M.; Ois, A.; Gomez Gonzalez, A.; Rodriguez-Campello, A.; de Ceballos, P.; Basagana, X.; Zabalza, A.; et al. Association of residential air pollution, noise, and greenspace with initial ischemic stroke severity. Environ. Res. 2019, 179, 108725. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Brook, J.R.; Goldberg, M.S.; Tu, K.; Copes, R.; Martin, R.V.; et al. Ambient Air Pollution and the Risk of Atrial Fibrillation and Stroke: A Population-Based Cohort Study. Environ. Health Perspect. 2019, 127, 87009. [Google Scholar] [CrossRef]
- Sun, S.; Stewart, J.D.; Eliot, M.N.; Yanosky, J.D.; Liao, D.; Tinker, L.F.; Eaton, C.B.; Whitsel, E.A.; Wellenius, G.A. Short-term exposure to air pollution and incidence of stroke in the Women’s Health Initiative. Environ. Int. 2019, 132, 105065. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, H.; Cai, B.; Fang, B.; Wang, C. Association between incidence of fatal intracerebral hemorrhagic stroke and fine particulate air pollution. Environ. Health Prev. Med. 2019, 24, 38. [Google Scholar] [CrossRef]
- Lee, K.K.; Miller, M.R.; Shah, A.S.V. Air Pollution and Stroke. J. Stroke 2018, 20, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, T.L.; Lin, Y.T.; Hwang, B.F.; Nakayama, S.F.; Tsai, C.H.; Sun, X.L.; Ma, C.; Jung, C.R. Fine particulate matter is a potential determinant of Alzheimer’s disease: A systemic review and meta-analysis. Environ. Res. 2019, 177, 108638. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K.K.L. The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Sci. Total Environ. 2019, 655, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Ljungman, P.L.S.; Eneroth, K.; Bellander, T.; Rizzuto, D. Association Between Cardiovascular Disease and Long-term Exposure to Air Pollution With the Risk of Dementia. JAMA Neurol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilango, S.D.; Chen, H.; Hystad, P.; van Donkelaar, A.; Kwong, J.C.; Tu, K.; Martin, R.V.; Benmarhnia, T. The role of cardiovascular disease in the relationship between air pollution and incident dementia: A population-based cohort study. Int. J. Epidemiol. 2020, 49, 36–44. [Google Scholar] [CrossRef]
- Cerza, F.; Renzi, M.; Gariazzo, C.; Davoli, M.; Michelozzi, P.; Forastiere, F.; Cesaroni, G. Long-term exposure to air pollution and hospitalization for dementia in the Rome longitudinal study. Environ. Health 2019, 18, 72. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Li, C.H.; Martini, S.; Hou, W.H. Association between air pollution and risk of vascular dementia: A multipollutant analysis in Taiwan. Environ. Int. 2019, 133, 105233. [Google Scholar] [CrossRef]
- Oudin, A.; Forsberg, B.; Adolfsson, A.N.; Lind, N.; Modig, L.; Nordin, M.; Nordin, S.; Adolfsson, R.; Nilsson, L.G. Traffic-Related Air Pollution and Dementia Incidence in Northern Sweden: A Longitudinal Study. Environ. Health Perspect. 2016, 124, 306–312. [Google Scholar] [CrossRef]
- Oudin, A.; Andersson, J.; Sundstrom, A.; Nordin Adolfsson, A.; Oudin Astrom, D.; Adolfsson, R.; Forsberg, B.; Nordin, M. Traffic-Related Air Pollution as a Risk Factor for Dementia: No Clear Modifying Effects of APOEvarepsilon4 in the Betula Cohort. J. Alzheimers Dis. 2019, 71, 733–740. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Hystad, P.; van Donkelaar, A.; Tu, K.; Brook, J.R.; Goldberg, M.S.; Martin, R.V.; Murray, B.J.; et al. Exposure to ambient air pollution and the incidence of dementia: A population-based cohort study. Environ. Int. 2017, 108, 271–277. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Andersson, J.; Oudin, A.; Sundstrom, A.; Forsberg, B.; Adolfsson, R.; Nordin, M. Road traffic noise, air pollution, and risk of dementia—results from the Betula project. Environ. Res. 2018, 166, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.M.; Anderson, H.R.; Atkinson, R.W.; Beevers, S.D.; Cook, D.G.; Strachan, D.P.; Dajnak, D.; Gulliver, J.; Kelly, F.J. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 2018, 8, e022404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Lu, Y.; Cheng, H.; Wang, C.; Chan, P. The impact of long-term exposure to ambient air pollution and second-hand smoke on the onset of Parkinson disease: A review and meta-analysis. Public Health 2020, 179, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Kasdagli, M.I.; Katsouyanni, K.; Dimakopoulou, K.; Samoli, E. Air pollution and Parkinson’s disease: A systematic review and meta-analysis up to 2018. Int. J. Hyg. Environ. Health 2019, 222, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Fang, Y.; Li, F.L.; Dong, B.; Hua, X.G.; Jiang, W.; Zhang, H.; Lyu, Y.; Zhang, X.J. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environ. Res. 2019, 168, 448–459. [Google Scholar] [CrossRef]
- Kulick, E.R.; Wellenius, G.A.; Boehme, A.K.; Joyce, N.R.; Schupf, N.; Kaufman, J.D.; Mayeux, R.; Sacco, R.L.; Manly, J.J.; Elkind, M.S.V. Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 2020, 94, e1782–e1792. [Google Scholar] [CrossRef]
- Lo, Y.C.; Lu, Y.C.; Chang, Y.H.; Kao, S.; Huang, H.B. Air Pollution Exposure and Cognitive Function in Taiwanese Older Adults: A Repeated Measurement Study. Int. J. Environ. Res. Public Health 2019, 16, 2976. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, X.; Zhang, X. The impact of exposure to air pollution on cognitive performance. Proc. Natl. Acad. Sci. USA 2018, 115, 9193–9197. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Noh, J.; Noh, Y.; Oh, S.S.; Koh, S.B.; Kim, C. Gender Difference in the Effects of Outdoor Air Pollution on Cognitive Function Among Elderly in Korea. Front. Public Health 2019, 7, 375. [Google Scholar] [CrossRef]
- Ailshire, J.; Karraker, A.; Clarke, P. Neighborhood social stressors, fine particulate matter air pollution, and cognitive function among older U.S. adults. Soc. Sci. Med. 2017, 172, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, N.M.; Henderson, V.W.; Hodis, H.N.; St John, J.A.; Lurmann, F.; Chen, J.C.; Mack, W.J. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 2014, 40, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonne, C.; Elbaz, A.; Beevers, S.; Singh-Manoux, A. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 2014, 25, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ailshire, J.A.; Crimmins, E.M. Fine particulate matter air pollution and cognitive function among older US adults. Am. J. Epidemiol. 2014, 180, 359–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ailshire, J.A.; Clarke, P. Fine particulate matter air pollution and cognitive function among U.S. older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2015, 70, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, M.C.; Weisskopf, M.G.; Alexeeff, S.E.; Coull, B.A.; Spiro, A., 3rd; Schwartz, J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ. Health Perspect. 2011, 119, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bertisch, S.M.; Mostofsky, E.; Buettner, C.; Mittleman, M.A. Weather, ambient air pollution, and risk of migraine headache onset among patients with migraine. Environ. Int. 2019, 132, 105100. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Cakmak, S.; Vidal, C.B. Air pollution and hospitalization for headache in Chile. Am. J. Epidemiol. 2009, 170, 1057–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyszkowicz, M.; Stieb, D.M.; Rowe, B.H. Air pollution and daily ED visits for migraine and headache in Edmonton, Canada. Am. J. Emerg. Med. 2009, 27, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Szyszkowicz, M. Ambient air pollution and daily emergency department visits for headache in Ottawa, Canada. Headache 2008, 48, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Szyszkowicz, M.; Rowe, B.H.; Kaplan, G.G. Ambient sulphur dioxide exposure and emergency department visits for migraine in Vancouver, Canada. Int. J. Occup. Med. Environ. Health 2009, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Szyszkowicz, M.; Kaplan, G.G.; Grafstein, E.; Rowe, B.H. Emergency department visits for migraine and headache: A multi-city study. Int. J. Occup. Med. Environ. Health 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.F.; Weng, Y.H.; Chiu, Y.W.; Yang, C.Y. Air pollution and daily clinic visits for headache in a subtropical city: Taipei, Taiwan. Int. J. Environ. Res. Public Health 2015, 12, 2277–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Chiu, H.F.; Yang, C.Y. Fine particulate air pollution and outpatient department visits for headache in Taipei, Taiwan. J. Toxicol. Environ. Health A 2015, 78, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Vodonos, A.; Novack, V.; Zlotnik, Y.; Ifergane, G. Ambient air pollution, weather and daily emergency department visits for headache. Cephalalgia 2015, 35, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Tian, X.; Yang, C.; Li, Y.; Hu, Y. Association between ambient air pollution and hospital admission for epilepsy in Eastern China. Epilepsy Res. 2019, 152, 52–58. [Google Scholar] [CrossRef]
- Xu, C.; Fan, Y.N.; Kan, H.D.; Chen, R.J.; Liu, J.H.; Li, Y.F.; Zhang, Y.; Ji, A.L.; Cai, T.J. The Novel Relationship between Urban Air Pollution and Epilepsy: A Time Series Study. PLoS ONE 2016, 11, e0161992. [Google Scholar] [CrossRef]
- Cakmak, S.; Dales, R.E.; Vidal, C.B. Air pollution and hospitalization for epilepsy in Chile. Environ. Int. 2010, 36, 501–505. [Google Scholar] [CrossRef]
- Costa, L.G.; Chang, Y.C.; Cole, T.B. Developmental Neurotoxicity of Traffic-Related Air Pollution: Focus on Autism. Curr. Environ. Health Rep. 2017, 4, 156–165. [Google Scholar] [CrossRef]
- Lam, J.; Sutton, P.; Kalkbrenner, A.; Windham, G.; Halladay, A.; Koustas, E.; Lawler, C.; Davidson, L.; Daniels, N.; Newschaffer, C.; et al. A Systematic Review and Meta-Analysis of Multiple Airborne Pollutants and Autism Spectrum Disorder. PLoS ONE 2016, 11, e0161851. [Google Scholar] [CrossRef] [Green Version]
- Flores-Pajot, M.C.; Ofner, M.; Do, M.T.; Lavigne, E.; Villeneuve, P.J. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environ. Res. 2016, 151, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Leung, C.; Wen, S.W.; McDonald, J.; Shin, H.H. Maternal exposure to air pollution and risk of autism in children: A systematic review and meta-analysis. Environ. Pollut. 2020, 256, 113307. [Google Scholar] [CrossRef] [PubMed]
- Pagalan, L.; Bickford, C.; Weikum, W.; Lanphear, B.; Brauer, M.; Lanphear, N.; Hanley, G.E.; Oberlander, T.F.; Winters, M. Association of Prenatal Exposure to Air Pollution With Autism Spectrum Disorder. JAMA Pediatr. 2019, 173, 86–92. [Google Scholar] [CrossRef]
- Jo, H.; Eckel, S.P.; Chen, J.C.; Cockburn, M.; Martinez, M.P.; Chow, T.; Lurmann, F.W.; Funk, W.E.; Xiang, A.H.; McConnell, R. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environ. Int. 2019, 133, 105110. [Google Scholar] [CrossRef] [PubMed]
- Oudin, A.; Frondelius, K.; Haglund, N.; Kallen, K.; Forsberg, B.; Gustafsson, P.; Malmqvist, E. Prenatal exposure to air pollution as a potential risk factor for autism and ADHD. Environ. Int. 2019, 133, 105149. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Liew, Z.; Yan, Q.; Cui, X.; Virk, J.; Ketzel, M.; Raaschou-Nielsen, O. Air pollution and Autism in Denmark. Environ. Epidemiol. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Raz, R.; Levine, H.; Pinto, O.; Broday, D.M.; Yuval; Weisskopf, M.G. Traffic-Related Air Pollution and Autism Spectrum Disorder: A Population-Based Nested Case-Control Study in Israel. Am. J. Epidemiol. 2018, 187, 717–725. [Google Scholar] [CrossRef]
- Gong, T.; Dalman, C.; Wicks, S.; Dal, H.; Magnusson, C.; Lundholm, C.; Almqvist, C.; Pershagen, G. Perinatal Exposure to Traffic-Related Air Pollution and Autism Spectrum Disorders. Environ. Health Perspect. 2017, 125, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Jin, Z.; Li, S.; Jin, X.; Tong, S.; Liu, S.; Yang, Y.; Huang, H.; Guo, Y. Early life exposure to particulate matter air pollution (PM1, PM2.5 and PM10) and autism in Shanghai, China: A case-control study. Environ. Int. 2018, 121, 1121–1127. [Google Scholar] [CrossRef]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Air pollution and newly diagnostic autism spectrum disorders: A population-based cohort study in Taiwan. PLoS ONE 2013, 8, e75510. [Google Scholar] [CrossRef] [Green Version]
- Yousefian, F.; Mahvi, A.H.; Yunesian, M.; Hassanvand, M.S.; Kashani, H.; Amini, H. Long-term exposure to ambient air pollution and autism spectrum disorder in children: A case-control study in Tehran, Iran. Sci. Total Environ. 2018, 643, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Kioumourtzoglou, M.A.; Hart, J.E.; Okereke, O.I.; Laden, F.; Weisskopf, M.G. The relation between past exposure to fine particulate air pollution and prevalent anxiety: Observational cohort study. BMJ 2015, 350, h1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pun, V.C.; Manjourides, J.; Suh, H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ. Health Perspect. 2017, 125, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braithwaite, I.; Zhang, S.; Kirkbride, J.B.; Osborn, D.P.J.; Hayes, J.F. Air Pollution (Particulate Matter) Exposure and Associations with Depression, Anxiety, Bipolar, Psychosis and Suicide Risk: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2019, 127, 126002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Liu, Q.; Deng, F.; Wang, X.; Lin, H.; Guo, X.; Wu, S. Association between particulate matter air pollution and risk of depression and suicide: Systematic review and meta-analysis. Br. J. Psychiatry 2019, 215, 456–467. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, R.; Liu, L.; Liu, Y.; Li, Y. Ambient air pollution exposure and risk of depression: A systematic review and meta-analysis of observational studies. Psychiatry Res. 2019, 276, 69–78. [Google Scholar] [CrossRef]
- Fan, S.J.; Heinrich, J.; Bloom, M.S.; Zhao, T.Y.; Shi, T.X.; Feng, W.R.; Sun, Y.; Shen, J.C.; Yang, Z.C.; Yang, B.Y.; et al. Ambient air pollution and depression: A systematic review with meta-analysis up to 2019. Sci. Total Environ. 2020, 701, 134721. [Google Scholar] [CrossRef]
- Newbury, J.B.; Arseneault, L.; Beevers, S.; Kitwiroon, N.; Roberts, S.; Pariante, C.M.; Kelly, F.J.; Fisher, H.L. Association of Air Pollution Exposure With Psychotic Experiences During Adolescence. JAMA Psychiatry 2019, 76, 614–623. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, X.; Zhang, Y.; Cheng, Q.; Duan, J.; Gao, J.; Xu, Z.; Zhang, H.; Wang, S.; Su, H. Ambient concentrations of NO2 and hospital admissions for schizophrenia. Occup. Environ. Med. 2019, 76, 125–131. [Google Scholar] [CrossRef]
- Croze, M.L.; Zimmer, L. Ozone Atmospheric Pollution and Alzheimer’s Disease: From Epidemiological Facts to Molecular Mechanisms. J. Alzheimers Dis. 2018, 62, 503–522. [Google Scholar] [CrossRef]
- Allen, J.L.; Klocke, C.; Morris-Schaffer, K.; Conrad, K.; Sobolewski, M.; Cory-Slechta, D.A. Cognitive Effects of Air Pollution Exposures and Potential Mechanistic Underpinnings. Curr. Environ. Health Rep. 2017, 4, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Pelch, K.E.; Bolden, A.L.; Kwiatkowski, C.F. Environmental Chemicals and Autism: A Scoping Review of the Human and Animal Research. Environ. Health Perspect. 2019, 127, 46001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.L.; Oberdorster, G.; Morris-Schaffer, K.; Wong, C.; Klocke, C.; Sobolewski, M.; Conrad, K.; Mayer-Proschel, M.; Cory-Slechta, D.A. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 2017, 59, 140–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Li, B.; Miao, J.J.; Yun, Y.; Li, G.K.; Sang, N. Seasonal variation in air particulate matter (PM10) exposure-induced ischemia-like injuries in the rat brain. Chem. Res. Toxicol. 2015, 28, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Yun, Y.; Li, H.; Hou, L.; Han, M.; Li, G. SO2 inhalation contributes to the development and progression of ischemic stroke in the brain. Toxicol. Sci. 2010, 114, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.; Anwar, K.; Ashraf, M. MRI and neuropathological validations of the involvement of air pollutants in cortical selective neuronal loss. Environ. Sci. Pollut. Res. Int. 2014, 21, 3351–3362. [Google Scholar] [CrossRef]
- Liu, Q.; Babadjouni, R.; Radwanski, R.; Cheng, H.; Patel, A.; Hodis, D.M.; He, S.; Baumbacher, P.; Russin, J.J.; Morgan, T.E.; et al. Stroke Damage Is Exacerbated by Nano-Size Particulate Matter in a Mouse Model. PLoS ONE 2016, 11, e0153376. [Google Scholar] [CrossRef]
- Morgan, T.E.; Davis, D.A.; Iwata, N.; Tanner, J.A.; Snyder, D.; Ning, Z.; Kam, W.; Hsu, Y.T.; Winkler, J.W.; Chen, J.C.; et al. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ. Health Perspect. 2011, 119, 1003–1009. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, Q.; Zhang, X.; Wu, S.; Wang, S.; Chen, R.; Li, X. Role of astrocyte activation in fine particulate matter-enhancement of existing ischemic stroke in Sprague-Dawley male rats. J. Toxicol. Environ. Health. A 2016, 79, 393–401. [Google Scholar] [CrossRef]
- Hullmann, M.; Albrecht, C.; van Berlo, D.; Gerlofs-Nijland, M.E.; Wahle, T.; Boots, A.W.; Krutmann, J.; Cassee, F.R.; Bayer, T.A.; Schins, R.P.F. Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part. Fibre Toxicol. 2017, 14, 35. [Google Scholar] [CrossRef]
- Woodward, N.C.; Levine, M.C.; Haghani, A.; Shirmohammadi, F.; Saffari, A.; Sioutas, C.; Morgan, T.E.; Finch, C.E. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J. Neuroinflamm. 2017, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yun, Y.; Ku, T.; Li, G.; Sang, N. NO2 inhalation promotes Alzheimer’s disease-like progression: Cyclooxygenase-2-derived prostaglandin E2 modulation and monoacylglycerol lipase inhibition-targeted medication. Sci. Rep. 2016, 6, 22429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durga, M.; Devasena, T.; Rajasekar, A. Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles. Environ. Toxicol. Pharmacol. 2015, 40, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garciduenas, L.; Herrera-Soto, A.; Jury, N.; Maher, B.A.; Gonzalez-Maciel, A.; Reynoso-Robles, R.; Ruiz-Rudolph, P.; van Zundert, B.; Varela-Nallar, L. Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution. Environ. Res. 2020, 183, 109226. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Morgan, T.E.; Saffari, A.A.; Shirmohammadi, F.; Forman, H.J.; Sioutas, C.; Finch, C.E. Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free. Radic. Biol. Med. 2020, 147, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Liu, X.; Weston, D.; Conrad, K.; Oberdorster, G.; Cory-Slechta, D.A. Consequences of developmental exposure to concentrated ambient ultrafine particle air pollution combined with the adult paraquat and maneb model of the Parkinson’s disease phenotype in male mice. Neurotoxicology 2014, 41, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Jew, K.; Herr, D.; Wong, C.; Kennell, A.; Morris-Schaffer, K.; Oberdorster, G.; O’Banion, M.K.; Cory-Slechta, D.A.; Elder, A. Selective memory and behavioral alterations after ambient ultrafine particulate matter exposure in aged 3xTgAD Alzheimer’s disease mice. Part. Fibre Toxicol. 2019, 16, 45. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Allen, J.L.; Conrad, K.; Marvin, E.; Sobolewski, M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology 2018, 69, 217–231. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Li, B.; Yang, H.; Cui, J.; Li, X.; Zhang, X.; Sun, H.; Meng, Q.; Wu, S.; et al. Activation of NLRP3 in microglia exacerbates diesel exhaust particles-induced impairment in learning and memory in mice. Environ. Int. 2020, 136, 105487. [Google Scholar] [CrossRef]
- Wang, F.; Fangfang, Z.; Guo, X.; Chen, W.; Yao, W.; Liu, H.; Lyu, C.; Zhang, Y.; Fan, C. Effects of volatile organic compounds and carbon monoxide mixtures on learning and memory, oxidative stress, and monoamine neurotransmitters in the brains of mice. Toxicol. Ind. Health 2018, 34, 178–187. [Google Scholar] [CrossRef]
- Ku, T.; Ji, X.; Zhang, Y.; Li, G.; Sang, N. PM2.5, SO2 and NO2 co-exposure impairs neurobehavior and induces mitochondrial injuries in the mouse brain. Chemosphere 2016, 163, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Win-Shwe, T.T.; Fujitani, Y.; Kyi-Tha-Thu, C.; Furuyama, A.; Michikawa, T.; Tsukahara, S.; Nitta, H.; Hirano, S. Effects of diesel engine exhaust origin secondary organic aerosols on novel object recognition ability and maternal behavior in BALB/c mice. Int. J. Environ. Res. Public Health 2014, 11, 11286–11307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, A.; Liu, H.; Salim, S. Involvement of oxidative stress and mitochondrial mechanisms in air pollution-related neurobiological impairments. Neurobiol. Stress 2020, 12, 100205. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, H.; Cui, S.; Han, B.; Zhou, L.; Zhang, N.; Su, X.; Niu, Y.; Chen, W.; Chen, R.; et al. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard Mater. 2019, 369, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Salvi, A.; Patki, G.; Liu, H.; Salim, S. Psychological Impact of Vehicle Exhaust Exposure: Insights from an Animal Model. Sci. Rep. 2017, 7, 8306. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.L.; Harvey, B.H.; Viljoen, F.; Ellis, S.M.; Brink, C.B. Ozone exposure of Flinders Sensitive Line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology 2015, 232, 2921–2938. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Liu, X.; Pelkowski, S.; Palmer, B.; Conrad, K.; Oberdorster, G.; Weston, D.; Mayer-Proschel, M.; Cory-Slechta, D.A. Early postnatal exposure to ultrafine particulate matter air pollution: Persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ. Health Perspect. 2014, 122, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Suwannasual, U.; Lucero, J.; McDonald, J.D.; Lund, A.K. Exposure to traffic-generated air pollutants mediates alterations in brain microvascular integrity in wildtype mice on a high-fat diet. Environ. Res. 2018, 160, 449–461. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Brown, G.C.; Vilalta, A. How microglia kill neurons. Brain Res. 2015, 1628, 288–297. [Google Scholar] [CrossRef]
- Haslund-Vinding, J.; McBean, G.; Jaquet, V.; Vilhardt, F. NADPH oxidases in oxidant production by microglia: Activating receptors, pharmacology and association with disease. Br. J. Pharmacol. 2017, 174, 1733–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBean, G.J.; Lopez, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef] [Green Version]
- Levesque, S.; Taetzsch, T.; Lull, M.E.; Johnson, J.A.; McGraw, C.; Block, M.L. The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. J. Neurochem. 2013, 125, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004, 18, 1618–1620. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Rathore, F.A. Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, R.; Long, B.; Gottlieb, M. Neurologic complications of COVID-19. Am. J. Emerg. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Scullen, T.; Mathkour, M.; Zeoli, T.; Beighley, A.; Kilgore, M.D.; Carr, C.; Zweifler, R.M.; Aysenne, A.; Maulucci, C.M.; et al. Neurological Impact of Coronavirus Disease (COVID-19): Practical Considerations for the Neuroscience Community. World Neurosurg. 2020. [Google Scholar] [CrossRef]
- Ogen, Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020, 726, 138605. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and covid-19 infection: Evidence from china. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef]





| Studies and Major Outcomes | Ref. |
|---|---|
| Stroke (cerebral ischemia) | |
| Seasonal variation in air particulate matter (PM10) exposure-induced ischemia-like injuries in the rat brain was attributed to varying toxin (PAHs) loading of the particles | [118] |
| SO2 inhalation contributes to the development and progression of ischemic stroke in the rat brain by enhanced endothelin-1 activity and inflammation (iNOS, COX-2, and ICAM-1 mRNA) followed by activation of caspase-3 and higher cerebral infarct volume | [119] |
| Air pollutants (PM generated by different engines and aluminum sulfate aerosols) caused cortical selective neuronal loss, nuclear pyknosis, karyolysis and karyorrhexis as well as activation of microglia and astrocytes (also features of stroke and other neurological disease) as revealed by magnetic resonance imaging | [120] |
| Stroke damage is aggravated by nano-size particulate matter in mice, secondary to more pronounced DNA damage (8-hydroxyguanosine) and oxidative stress (gp91phox, p47phox) as well as higher number of inflammatory cells (CD68 and Ly6g positive) | [121] |
| Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants (PM0.2) in mice, suggesting additive effects of air pollution and ischemic stroke on cerebral damage | [122] |
| Astrocyte activation plays a role in fine particulate matter (PM2.5)-dependent aggravation of ischemic stroke in male rats | [123] |
| Dementia (Alzheimer’s disease) | |
| Diesel engine exhaust accelerates amyloid β42 plaque formation in the 5X Familial AD mouse model of Alzheimer’s disease, although no additive effects on spatial working memory deficits (assessed by Y-maze and X-maze tests) and markers of inflammation (IL-1β, RANTES and MCP-1) were observed | [124] |
| Central role of Toll-like receptor 4 for glial inflammatory responses (higher TLR4, MyD88, TNFα, and TNFR2 mRNA) to air pollution (PM0.2) in rats leading to a neuroinflammatory, accelerated cognitive aging and dementia-like phenotype | [125] |
| NO2 inhalation promotes Alzheimer’s disease-like progression via cyclooxygenase-2-derived prostaglandin E2 modulation, altered astrocyte and microglia function, all of which leading to deterioration of spatial learning and memory as well as aggravated amyloid β42 accumulation in wildtype C57BL/6J or Alzheimer’s disease-prone APP/PS1 mice | [126] |
| Neurotoxicity of diesel exhaust nanoparticles in the rat brain is associated with increased levels of pro-inflammatory cytokines, amyloid β42, reactive oxygen species, hydrogen peroxide, nitrogen oxide metabolites and apurinic/apyrimidinic sites (DNA damage) | [127] |
| Exposure of mice to particulate urban air pollution reduced the repressive epigenetic marks (H3K9me2/me3) and increased DNA damage (γ-H2AX) as well as Alzheimer’s disease hallmarks (hyperphosphorylated tau and amyloid-β plaques) in the brain | [128] |
| Traffic-related air pollutants (nano-sized PM) promote neuronal amyloidogenesis (amyloid-β deposition) through oxidative damage (4-HNE, 3NT) in lipid rafts of mice | [129] |
| Parkinson’s disease | |
| Developmental exposure to concentrated ambient ultrafine particle air pollution (similar to the paraquat and maneb model) cause a Parkinson’s disease phenotype in male mice with locomotor dysfunction and dopaminergic and glutamatergic changes | [130] |
| Cognitive and memory impairment | |
| Selective memory and behavioral alterations after ambient ultrafine particulate matter exposure (using the Harvard ultrafine concentrated ambient particle system) in aged 3xTgAD Alzheimer’s disease mice | [131] |
| Developmental exposure to low level concentrated ambient ultrafine particle air pollution and cognitive dysfunction in mice revealed by complementary learning (repeated learning), memory (novel object recognition, NOR), impulsive-like behavior (differential reinforcement of low rate (DRL), schedule of reward and delay of reward (DOR)), motor activity (locomotor behavior) and motivation (progressive ratio schedule) assessment assays | [132] |
| Activation of NLRP3 in microglia exacerbates diesel exhaust particles-induced impairment in learning and memory in mice | [133] |
| Impairment of learning and memory, induction of oxidative stress and dysregulation of monoamine neurotransmitters in the brains of mice by exposure to volatile organic compounds and carbon monoxide mixtures | [134] |
| PM2.5, SO2 and NO2 co-exposure impairs neurobehavior and induces mitochondrial injuries in the mouse brain | [135] |
| Effects of diesel engine exhaust origin secondary organic aerosols on novel object recognition ability and maternal behavior in mice | [136] |
| Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal long-term potentiation by increasing brain inflammation and oxidative stress in rats | [30] |
| Mental disorders | |
| Involvement of oxidative stress and mitochondrial mechanisms in air pollution (simulated vehicle exhaust)-related neurobiological impairments in rats leading to anxiety- and depression-like behavior | [137] |
| Ambient PM2.5 exposure caused depressive-like responses in mice through Nrf2/NLRP3 signaling pathway and altered inflammation | [138] |
| Psychological impact of vehicle exhaust exposure (CO, CO2, NO2) as revealed by anxiety- and depression-like behavior as well as impaired memory in rats | [139] |
| Ozone exposure of rats (Flinders Sensitive Line translational model) caused neurobiological oxidative stress and a depression-like phenotype | [140] |
| Other adverse effects on the brain (e.g., impaired BBB) | |
| Early postnatal exposure to ultrafine particulate matter air pollution leads to dysregulated CNS neurotransmitters, cytokines and glial activation preferentially in male mice. In addition, lateral ventricle dilation (=ventriculomegaly) was observed in exposed male mice, which is associated with poor neurodevelopmental outcome, autism, and schizophrenia | [141] |
| Exposure to traffic-generated air pollutants mediates alterations in brain microvascular integrity (disrupted blood-brain barrier) and enhanced oxidized low density lipoprotein signaling in wildtype mice on a high-fat diet, indicating additive adverse effects of obesity and air pollution on brain function | [142] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. https://doi.org/10.3390/ijms21124306
Hahad O, Lelieveld J, Birklein F, Lieb K, Daiber A, Münzel T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. International Journal of Molecular Sciences. 2020; 21(12):4306. https://doi.org/10.3390/ijms21124306
Chicago/Turabian StyleHahad, Omar, Jos Lelieveld, Frank Birklein, Klaus Lieb, Andreas Daiber, and Thomas Münzel. 2020. "Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress" International Journal of Molecular Sciences 21, no. 12: 4306. https://doi.org/10.3390/ijms21124306