The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion
Abstract
:1. Introduction
2. The Biological Functions of Cell Adhesion
3. Role of Major Adhesion Molecules in Cell Adhesion
3.1. Integrins
3.2. Immunoglobulin Superfamily
3.2.1. ICAM-1
3.2.2. VCAM-1
3.2.3. PECAM-1
3.3. Cadherins
3.4. Selectin
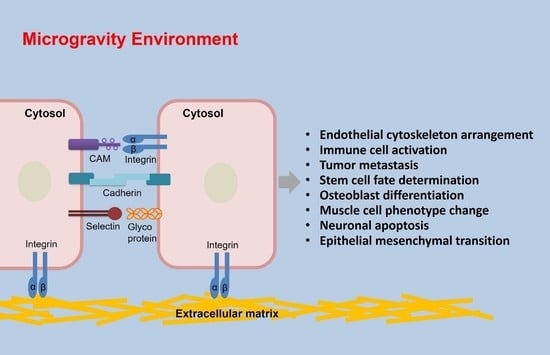
4. Effects of Microgravity on Cell Adhesion
4.1. Microgravity Regulates Adhesion and Cytoskeleton Arrangement of Endothelial Cells
4.2. Microgravity Regulates Adhesion and Activation of Immune Cells
4.3. Microgravity Inhibits Tumor Cells Adhesion
4.4. Microgravity Regulates Adhesion and Fate Determination of Stem Cells
4.5. Microgravity Inhibits Osteoblast Adhesion and Differentiation
4.6. Microgravity Regulates the Adhesion and Phenotype of Muscle Cells
4.7. The Function of Microgravity on Adhesion of Other Cell Types
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAM | Cell adhesion molecules |
| ECM | Extracellular matrix |
| Ig-SF | Immunoglobulin superfamily |
| ISS | International space station |
| VE-cadherin | Vascular endothelial cadherin |
| FAK | Focal adhesion kinase |
| YAP | Yes-associated protein |
| ICAM | Intercellular adhesion molecule |
| VCAM | Vascular cell adhesion molecule |
| PECAM | Platelet endothelial cell adhesion molecule |
| EPCs | Endothelial progenitor cells |
| MSCs | Mesenchymal stem cells |
| FGFR | Fibroblast growth factor receptor |
| HUVEC | Human umbilical vein endothelial cells |
| RPM | Random positioning machine |
| HLU | Hindlimb unweighted |
| RCCS | Rotary cell culture system |
| MCP-1 | Monocyte chemoattractant protein |
| HLA-DR | Major histocompatibility complex, class II, DR |
| PYK2 | Proline-rich tyrosine kinase 2 |
| ADSCs | Adipose-derived stem cells |
| OPN | Osteopontin |
| VSMCs | Vascular smooth muscle cells |
| SMPCs | Skeletal muscle stem/progenitor cells |
| LAMB2 | Laminin subunit β-2 |
| NHDF | Human dermal fibroblasts |
References
- Aleshcheva, G.; Bauer, J.; Hemmersbach, R.; Slumstrup, L.; Wehland, M.; Infanger, M.; Grimm, D. Scaffold-free Tissue Formation Under Real and Simulated Microgravity Conditions. Basic Clin. Pharmacol. Toxicol. 2016, 119 (Suppl. S3), 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anil-Inevi, M.; Yilmaz, E.; Sarigil, O.; Tekin, H.C.; Ozcivici, E. Single Cell Densitometry and Weightlessness Culture of Mesenchymal Stem Cells Using Magnetic Levitation. Methods Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Tanaka, K.; Nishimura, N.; Kawai, Y. Adaptation to microgravity, deconditioning, and countermeasures. J. Physiol. Sci. 2017, 67, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.M.; Leckband, D.; Yap, A.S. Tissue organization by cadherin adhesion molecules: Dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol. Rev. 2011, 91, 691–731. [Google Scholar] [CrossRef] [Green Version]
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 2011, 124, 1183–1193. [Google Scholar] [CrossRef] [Green Version]
- Desai, R.A.; Gao, L.; Raghavan, S.; Liu, W.F.; Chen, C.S. Cell polarity triggered by cell-cell adhesion via E-cadherin. J. Cell Sci. 2009, 122, 905–911. [Google Scholar] [CrossRef] [Green Version]
- Jhala, D.V.; Kale, R.K.; Singh, R.P. Microgravity alters cancer growth and progression. Curr. Cancer Drug Targets 2014, 14, 394–406. [Google Scholar] [CrossRef]
- Singh, J.; Hussain, F.; Decuzzi, P. Role of differential adhesion in cell cluster evolution: From vasculogenesis to cancer metastasis. Comput. Methods Biomech. Biomed. Engin. 2015, 18, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Masiello, M.G.; Verna, R.; Cucina, A.; Bizzarri, M. Physical constraints in cell fate specification. A case in point: Microgravity and phenotypes differentiation. Prog. Biophys. Mol. Biol. 2018, 134, 55–67. [Google Scholar] [CrossRef]
- Bajanca, F.; Alfandari, D.; Thorsteinsdottir, S.; Theveneau, E. Editorial: Cell adhesion in development. Dev. Biol. 2015, 401, 1. [Google Scholar] [CrossRef] [Green Version]
- Marjoram, R.J.; Lessey, E.C.; Burridge, K. Regulation of RhoA activity by adhesion molecules and mechanotransduction. Curr. Mol. Med. 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimmel, L.; Gordon, E. The precise molecular signals that control endothelial cell-cell adhesion within the vessel wall. Biochem. Soc. Trans. 2018, 46, 1673–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeliet, P.; Lampugnani, M.G.; Moons, L.; Breviario, F.; Compernolle, V.; Bono, F.; Balconi, G.; Spagnuolo, R.; Oosthuyse, B.; Dewerchin, M.; et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 1999, 98, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Hariharan, V.; Huang, H. Cell-cell contact preserves cell viability via plakoglobin. PLoS ONE 2011, 6, e27064. [Google Scholar] [CrossRef] [Green Version]
- Peluso, J.J.; Pappalardo, A.; Trolice, M.P. N-cadherin-mediated cell contact inhibits granulosa cell apoptosis in a progesterone-independent manner. Endocrinology 1996, 137, 1196–1203. [Google Scholar] [CrossRef]
- Venhuizen, J.H.; Zegers, M.M. Making Heads or Tails of It: Cell-Cell Adhesion in Cellular and Supracellular Polarity in Collective Migration. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Barriga, E.H.; Mayor, R. Embryonic cell-cell adhesion: A key player in collective neural crest migration. Curr. Top. Dev. Biol. 2015, 112, 301–323. [Google Scholar] [CrossRef]
- Nardini, J.T.; Chapnick, D.A.; Liu, X.; Bortz, D.M. Modeling keratinocyte wound healing dynamics: Cell-cell adhesion promotes sustained collective migration. J. Theor. Biol. 2016, 400, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Maziveyi, M.; Alahari, S.K. Cell matrix adhesions in cancer: The proteins that form the glue. Oncotarget 2017, 8, 48471–48487. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, N.C.; Roca-Cusachs, P. Mechanosensing at integrin-mediated cell-matrix adhesions: From molecular to integrated mechanisms. Curr. Opin. Cell Biol. 2018, 50, 20–26. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A. Cell adhesion molecules: Potential therapeutic & diagnostic implications. Mol. Biotechnol. 2008, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tamkun, J.W.; DeSimone, D.W.; Fonda, D.; Patel, R.S.; Buck, C.; Horwitz, A.F.; Hynes, R.O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell 1986, 46, 271–282. [Google Scholar] [CrossRef]
- Hubbard, A.K.; Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 2000, 28, 1379–1386. [Google Scholar] [CrossRef]
- Lee, B.K.; Lee, W.J.; Jung, Y.S. Chrysin Attenuates VCAM-1 Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Preventing NF-kappaB Signaling. Int. J. Mol. Sci. 2017, 18, 1424. [Google Scholar] [CrossRef] [Green Version]
- Toma, L.; Sanda, G.M.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Glycated LDL increase VCAM-1 expression and secretion in endothelial cells and promote monocyte adhesion through mechanisms involving endoplasmic reticulum stress. Mol. Cell Biochem. 2016, 417, 169–179. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Liu, S.; Parajuli, K.R.; Qu, Y.; Mei, J.; Chen, Z.; Zhang, H.; Khismatullin, D.B.; You, Z. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate 2015, 75, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Coopman, P.; Djiane, A. Adherens Junction and E-Cadherin complex regulation by epithelial polarity. Cell Mol. Life Sci. 2016, 73, 3535–3553. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witz, I.P. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008, 27, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Angiari, S. Selectin-mediated leukocyte trafficking during the development of autoimmune disease. Autoimmun Rev. 2015, 14, 984–995. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [Green Version]
- Seetharaman, S.; Etienne-Manneville, S. Integrin diversity brings specificity in mechanotransduction. Biol. Cell 2018, 110, 49–64. [Google Scholar] [CrossRef]
- Iwamoto, D.V.; Calderwood, D.A. Regulation of integrin-mediated adhesions. Curr. Opin. Cell Biol. 2015, 36, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Matthaus, C.; Langhorst, H.; Schutz, L.; Juttner, R.; Rathjen, F.G. Cell-cell communication mediated by the CAR subgroup of immunoglobulin cell adhesion molecules in health and disease. Mol. Cell Neurosci. 2017, 81, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Malik, P.; Arora, S.K.; Mukherjee, T.K. Intercellular adhesion molecule-1 as a drug target in asthma and rhinitis. Respirology 2014, 19, 508–513. [Google Scholar] [CrossRef]
- Goh, Q.; Dearth, C.L.; Corbett, J.T.; Pierre, P.; Chadee, D.N.; Pizza, F.X. Intercellular adhesion molecule-1 expression by skeletal muscle cells augments myogenesis. Exp. Cell Res. 2015, 331, 292–308. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhang, H.; Xia, J.; Hou, J.; Wang, Y.; Yang, T.; Wang, S.; Zhang, X.; Chen, X.; Wu, X. Interleukin-1beta induces intercellular adhesion molecule-1 expression, thus enhancing the adhesion between mesenchymal stem cells and endothelial progenitor cells via the p38 MAPK signaling pathway. Int. J. Mol. Med. 2018, 41, 1976–1982. [Google Scholar] [CrossRef]
- Roland, C.L.; Dineen, S.P.; Toombs, J.E.; Carbon, J.G.; Smith, C.W.; Brekken, R.A.; Barnett, C.C., Jr. Tumor-derived intercellular adhesion molecule-1 mediates tumor-associated leukocyte infiltration in orthotopic pancreatic xenografts. Exp. Biol. Med. 2010, 235, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, M.; Bendas, G. Vascular cell adhesion molecule-1 (VCAM-1)--an increasing insight into its role in tumorigenicity and metastasis. Int. J. Cancer 2015, 136, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhang, X.H.; Massague, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Sharma, R.; Khaket, T.P.; Dutta, C.; Chakraborty, B.; Mukherjee, T.K. Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol. 2017, 40, 199–208. [Google Scholar] [CrossRef]
- Newman, P.J. The biology of PECAM-1. J. Clin. Investig. 1997, 100, S25–S29. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial PECAM-1 and its function in vascular physiology and atherogenic pathology. Exp. Mol. Pathol. 2016, 100, 409–415. [Google Scholar] [CrossRef]
- Chiba, R.; Nakagawa, N.; Kurasawa, K.; Tanaka, Y.; Saito, Y.; Iwamoto, I. Ligation of CD31 (PECAM-1) on endothelial cells increases adhesive function of alphavbeta3 integrin and enhances beta1 integrin-mediated adhesion of eosinophils to endothelial cells. Blood 1999, 94, 1319–1329. [Google Scholar] [CrossRef]
- Snyder, J.L.; McBeath, E.; Thomas, T.N.; Chiu, Y.J.; Clark, R.L.; Fujiwara, K. Mechanotransduction properties of the cytoplasmic tail of PECAM-1. Biol. Cell 2017, 109, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, A.; Shively, J.E. Angiopoietins-1 and -2 play opposing roles in endothelial sprouting of embryoid bodies in 3D culture and their receptor Tie-2 associates with the cell-cell adhesion molecule PECAM1. Exp. Cell Res. 2011, 317, 2171–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasch, J.; Harrison, O.J.; Honig, B.; Shapiro, L. Thinking outside the cell: How cadherins drive adhesion. Trends Cell Biol. 2012, 22, 299–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, K.H.; Zaidel-Bar, R. Early events in the assembly of E-cadherin adhesions. Exp. Cell Res. 2017, 358, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bruner, H.C.; Derksen, P.W.B. Loss of E-Cadherin-Dependent Cell-Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Derycke, L.D.; Bracke, M.E. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 2004, 48, 463–476. [Google Scholar] [CrossRef]
- Nguyen, T.; Mege, R.M. N-Cadherin and Fibroblast Growth Factor Receptors crosstalk in the control of developmental and cancer cell migrations. Eur. J. Cell Biol. 2016, 95, 415–426. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Corada, M.; Andriopoulou, P.; Esser, S.; Risau, W.; Dejana, E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J. Cell Sci. 1997, 110 Pt 17, 2065–2077. [Google Scholar]
- McEver, R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015, 107, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Jia, G.; Bao, J.; Zhang, Y.; Bai, Y.; Lin, L.; Tang, H.; Ma, J. Increased vascular cell adhesion molecule-1 was associated with impaired endothelium-dependent relaxation of cerebral and carotid arteries in simulated microgravity rats. J. Physiol. Sci. 2008, 58, 67–73. [Google Scholar] [CrossRef]
- Versari, S.; Longinotti, G.; Barenghi, L.; Maier, J.A.; Bradamante, S. The challenging environment on board the International Space Station affects endothelial cell function by triggering oxidative stress through thioredoxin interacting protein overexpression: The ESA-SPHINX experiment. FASEB J. 2013, 27, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lu, D.; Chen, Q.; Long, M. Microgravity-Induced Alterations of Inflammation-Related Mechanotransduction in Endothelial Cells on Board SJ-10 Satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittrich, A.; Grimm, D.; Sahana, J.; Bauer, J.; Kruger, M.; Infanger, M.; Magnusson, N.E. Key Proteins Involved in Spheroid Formation and Angiogenesis in Endothelial Cells After Long-Term Exposure to Simulated Microgravity. Cell Physiol. Biochem. 2018, 45, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ran, H.H.; Gao, Y.L.; Ma, J.; Huang, Y.; Bai, Y.G.; Lin, L.J. Differential vascular cell adhesion molecule-1 expression and superoxide production in simulated microgravity rat vasculature. EXCLI J. 2010, 9, 195–204. [Google Scholar]
- Xu, D.; Guo, Y.B.; Zhang, M.; Sun, Y.Q. The subsequent biological effects of simulated microgravity on endothelial cell growth in HUVECs. Chin. J. Traumatol. 2018, 21, 229–237. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Feiveson, A.H.; Krieger, S.; Kay Brinda, A.; von Scheven, G.; Burkle, A.; Crucian, B.; Wu, H. Synergistic Effects of Weightlessness, Isoproterenol, and Radiation on DNA Damage Response and Cytokine Production in Immune Cells. Int. J. Mol. Sci. 2018, 19, 3689. [Google Scholar] [CrossRef] [Green Version]
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 2017, 12, e0175599. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, K.; Tauber, S.; Dumrese, C.; Bradacs, G.; Simmet, D.M.; Golz, N.; Hauschild, S.; Raig, C.; Engeli, S.; Gutewort, A.; et al. Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity. Biomed. Res. Int. 2015, 2015, 538786. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.C.; Yue, Y.; Yu, J.W.; Cai, Y.; Bai, Y.G.; Zhang, H.J.; Bao, J.X.; Ren, X.L.; Xie, M.J.; et al. Simulated microgravity induces an inflammatory response in the common carotid artery of rats. Can. J. Physiol. Pharmacol. 2014, 92, 661–668. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.C.; Bai, Y.G.; Cai, Y.; Yu, J.W.; Zhang, H.J.; Bao, J.X.; Ren, X.L.; Xie, M.J.; Ma, J. Simulated microgravity promotes monocyte adhesion to rat aortic endothelium via nuclear factor-kappaB activation. Clin. Exp. Pharmacol. Physiol. 2015, 42, 510–519. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.; Quiriarte, H.; Pierson, D.; Sams, C. Monocyte phenotype and cytokine production profiles are dysregulated by short-duration spaceflight. Aviat Space Environ. Med. 2011, 82, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.; Sun, S.J.; Li, N.; Biere, K.; Hoerl, M.; Matzel, S.; Feuerecker, M.; Buchheim, J.I.; Strewe, C.; Thiel, C.S.; et al. Cells Flow and Immune Cell Priming under alternating g-forces in Parabolic Flight. Sci. Rep. 2019, 9, 11276. [Google Scholar] [CrossRef] [PubMed]
- Rossy, J.; Laufer, J.M.; Legler, D.F. Role of Mechanotransduction and Tension in T Cell Function. Front. Immunol. 2018, 9, 2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Li, R.; Tan, X.; Zhang, J.; Fan, C.; Zhao, Q.; Deng, Y.; Xu, A.; Lukong, K.E.; Genth, H.; et al. Simulated Microgravity Reduces Focal Adhesions and Alters Cytoskeleton and Nuclear Positioning Leading to Enhanced Apoptosis via Suppressing FAK/RhoA-Mediated mTORC1/NF-kappaB and ERK1/2 Pathways. Int. J. Mol. Sci. 2018, 19, 1994. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Liu, R.; Tian, X.; Han, Z.; Chen, J. Simulated microgravity inhibits the viability and migration of glioma via FAK/RhoA/Rock and FAK/Nek2 signaling. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 260–271. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, J.; Du, T.; Wang, Y.; Wang, Z. Modeled microgravity causes changes in the cytoskeleton and focal adhesions, and decreases in migration in malignant human MCF-7 cells. Protoplasma 2009, 238, 23–33. [Google Scholar] [CrossRef]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Kruger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K.; et al. Simulated Microgravity Influences VEGF, MAPK, and PAM Signaling in Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef] [Green Version]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis Induction and Alteration of Cell Adherence in Human Lung Cancer Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef] [Green Version]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Kruger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schutte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef] [Green Version]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Kruger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased E-Cadherin in MCF7 Human Breast Cancer Cells Forming Multicellular Spheroids Exposed to Simulated Microgravity. Proteomics 2018, 18, e1800015. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Kruger, M.; Bauer, J.; Wehland, M.; Corydon, T.J.; Sahana, J.; Nassef, M.Z.; Melnik, D.; Bauer, T.J.; Schulz, H.; et al. Microgravity Affects Thyroid Cancer Cells during the TEXUS-53 Mission Stronger than Hypergravity. Int. J. Mol. Sci. 2018, 19, 4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Kruger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratushnyy, A.Y.; Buravkova, L.B. Expression of focal adhesion genes in mesenchymal stem cells under simulated microgravity. Dokl. Biochem. Biophys. 2017, 477, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Gershovich, P.M.; Gershovich Iu, G.; Buravkova, L.B. Cytoskeleton structures and adhesion properties of human stromal precursors under conditions of simulated microgravity. Tsitologiia 2009, 51, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Koaykul, C.; Kim, M.H.; Kawahara, Y.; Yuge, L.; Kino-Oka, M. Alterations in Nuclear Lamina and the Cytoskeleton of Bone Marrow-Derived Human Mesenchymal Stem Cells Cultured Under Simulated Microgravity Conditions. Stem Cells Dev. 2019, 28, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sun, S.; Zhang, F.; Luo, C.; Zheng, L.; Wu, Y.; Li, N.; Zhang, C.; Wang, C.; Chen, Q.; et al. Microgravity-induced hepatogenic differentiation of rBMSCs on board the SJ-10 satellite. FASEB J. 2019, 33, 4273–4286. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Zhang, C.; Chen, G.; Tang, Z.; Liu, Q.; Chen, J.; Tong, X.; Wang, J. Effects of BMP-2 and FGF2 on the osteogenesis of bone marrow-derived mesenchymal stem cells in hindlimb-unloaded rats. Cell. Biochem. Biophys. 2014, 70, 1127–1136. [Google Scholar] [CrossRef]
- Meyers, V.E.; Zayzafoon, M.; Gonda, S.R.; Gathings, W.E.; McDonald, J.M. Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. J. Cell. Biochem. 2004, 93, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Ebnerasuly, F.; Hajebrahimi, Z.; Tabaie, S.M.; Darbouy, M. Simulated Microgravity Condition Alters the Gene Expression of some ECM and Adhesion Molecules in Adipose Derived Stem Cells. Int. J. Mol. Cell. Med. 2018, 7, 146–157. [Google Scholar] [CrossRef]
- Pisanu, M.E.; Noto, A.; De Vitis, C.; Masiello, M.G.; Coluccia, P.; Proietti, S.; Giovagnoli, M.R.; Ricci, A.; Giarnieri, E.; Cucina, A.; et al. Lung cancer stem cell lose their stemness default state after exposure to microgravity. Biomed. Res. Int. 2014, 2014, 470253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jiang, Y.; Wang, C.; Geng, B.; Wang, Y.; Chen, J.; Liu, F.; Qiu, P.; Zhai, G.; et al. Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J. 2018, 32, 4444–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kumei, Y.; Morita, S.; Katano, H.; Akiyama, H.; Hirano, M.; Oyha, K.; Shimokawa, H. Microgravity signal ensnarls cell adhesion, cytoskeleton, and matrix proteins of rat osteoblasts: Osteopontin, CD44, osteonectin, and alpha-tubulin. Ann. N. Y. Acad. Sci. 2006, 1090, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.; Grimm, D.; Corydon, T.J.; Kruger, M.; Wehland, M.; Riwaldt, S.; Sahana, J.; Kopp, S.; Bauer, J.; Reseland, J.E.; et al. Changes in Human Foetal Osteoblasts Exposed to the Random Positioning Machine and Bone Construct Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioia, M.; Michaletti, A.; Scimeca, M.; Marini, M.; Tarantino, U.; Zolla, L.; Coletta, M. Simulated microgravity induces a cellular regression of the mature phenotype in human primary osteoblasts. Cell. Death Discov. 2018, 4, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guignandon, A.; Akhouayri, O.; Usson, Y.; Rattner, A.; Laroche, N.; Lafage-Proust, M.H.; Alexandre, C.; Vico, L. Focal contact clustering in osteoblastic cells under mechanical stresses: Microgravity and cyclic deformation. Cell. Commun. Adhes. 2003, 10, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Guignandon, A.; Lafage-Proust, M.H.; Usson, Y.; Laroche, N.; Caillot-Augusseau, A.; Alexandre, C.; Vico, L. Cell cycling determines integrin-mediated adhesion in osteoblastic ROS 17/2.8 cells exposed to space-related conditions. FASEB J. 2001, 15, 2036–2038. [Google Scholar] [CrossRef] [Green Version]
- Guignandon, A.; Faure, C.; Neutelings, T.; Rattner, A.; Mineur, P.; Linossier, M.T.; Laroche, N.; Lambert, C.; Deroanne, C.; Nusgens, B.; et al. Rac1 GTPase silencing counteracts microgravity-induced effects on osteoblastic cells. FASEB J. 2014, 28, 4077–4087. [Google Scholar] [CrossRef] [Green Version]
- Saxena, R.; Pan, G.; McDonald, J.M. Osteoblast and osteoclast differentiation in modeled microgravity. Ann. N. Y. Acad. Sci. 2007, 1116, 494–498. [Google Scholar] [CrossRef]
- Philippou, A.; Minozzo, F.C.; Spinazzola, J.M.; Smith, L.R.; Lei, H.; Rassier, D.E.; Barton, E.R. Masticatory muscles of mouse do not undergo atrophy in space. FASEB J. 2015, 29, 2769–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Fan, Y.; Sun, A.; Jia, X.; Deng, X. Simulated microgravity exposure modulates the phenotype of cultured vascular smooth muscle cells. Cell. Biochem. Biophys. 2013, 66, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lyu, Q.; Bai, Y.G.; Liu, H.; Yang, J.; Cheng, J.H.; Zheng, M.; Ma, J. Focal adhesions are involved in simulated-microgravity-induced basilar and femoral arterial remodelling in rats. Can. J. Physiol. Pharmacol. 2018, 96, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, T.; Ichida, S.; Kanno, M.; Ishihara, R.; Hatashima, T.; Ueno, K.; Hamano, K. Microgravity influences maintenance of the human muscle stem/progenitor cell pool. Biochem. Biophys. Res. Commun. 2017, 493, 998–1003. [Google Scholar] [CrossRef]
- Berberoglu, M.A.; Gallagher, T.L.; Morrow, Z.T.; Talbot, J.C.; Hromowyk, K.J.; Tenente, I.M.; Langenau, D.M.; Amacher, S.L. Satellite-like cells contribute to pax7-dependent skeletal muscle repair in adult zebrafish. Dev. Biol. 2017, 424, 162–180. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Wang, D.; Zeng, F.; Wei, Y.; Wang, F.; Feng, C.; Li, N.; Dai, R.; Deng, Y.; et al. Effects of simulated microgravity on human brain nervous tissue. Neurosci. Lett. 2016, 627, 199–204. [Google Scholar] [CrossRef]
- Corydon, T.J.; Mann, V.; Slumstrup, L.; Kopp, S.; Sahana, J.; Askou, A.L.; Magnusson, N.E.; Echegoyen, D.; Bek, T.; Sundaresan, A.; et al. Reduced Expression of Cytoskeletal and Extracellular Matrix Genes in Human Adult Retinal Pigment Epithelium Cells Exposed to Simulated Microgravity. Cell. Physiol. Biochem. 2016, 40, 1–17. [Google Scholar] [CrossRef]
- Ranieri, D.; Proietti, S.; Dinicola, S.; Masiello, M.G.; Rosato, B.; Ricci, G.; Cucina, A.; Catizone, A.; Bizzarri, M.; Torrisi, M.R. Simulated microgravity triggers epithelial mesenchymal transition in human keratinocytes. Sci. Rep. 2017, 7, 538. [Google Scholar] [CrossRef]
- Buken, C.; Sahana, J.; Corydon, T.J.; Melnik, D.; Bauer, J.; Wehland, M.; Kruger, M.; Balk, S.; Abuagela, N.; Infanger, M.; et al. Morphological and Molecular Changes in Juvenile Normal Human Fibroblasts Exposed to Simulated Microgravity. Sci. Rep. 2019, 9, 11882. [Google Scholar] [CrossRef]
- Shi, S.; Li, Q.; Cao, Q.; Diao, Y.; Zhang, Y.; Yue, L.; Wei, L. EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin. Int. J. Mol. Sci. 2020, 21, 1349. [Google Scholar] [CrossRef] [Green Version]
- Bauer, T.J.; Gombocz, E.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. Insight in Adhesion Protein Sialylation and Microgravity Dependent Cell Adhesion-An Omics Network Approach. Int. J. Mol. Sci. 2020, 21, 1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romswinkel, A.; Infanger, M.; Dietz, C.; Strube, F.; Kraus, A. The Role of C-X-C Chemokine Receptor Type 4 (CXCR4) in Cell Adherence and Spheroid Formation of Human Ewing’s Sarcoma Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 6073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolovskaya, A.; Korneeva, E.; Zaichenko, D.; Virus, E.; Kolesov, D.; Moskovtsev, A.; Kubatiev, A. Changes in the Surface Expression of Intercellular Adhesion Molecule 3, the Induction of Apoptosis, and the Inhibition of Cell-Cycle Progression of Human Multidrug-Resistant Jurkat/A4 Cells Exposed to a Random Positioning Machine. Int. J. Mol. Sci. 2020, 21, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennarini, G.; Furley, A. Cell adhesion molecules in neural development and disease. Mol. Cell. Neurosci. 2017, 81, 1–3. [Google Scholar] [CrossRef]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef]
| Adhesion Molecules | Classification | Functions in Cell Adhesion | References |
|---|---|---|---|
| Integrins | 24 known αβ-heterodimers, 18 α-subunits, and 8 β-subunits | Connection between the extracellular matrix (ECM) and the actin cytoskeleton of cells | [24] |
| Immunoglobulin superfamily (Ig-SF) | Intercellular adhesion molecule (ICAM-1) | Cell–cell adhesion by binding to specific ligands in the ECM and surrounding cells | [25] |
| Vascular cell adhesion molecule (VCAM-1) | Leukocyte adhesion to endothelial cells | [26,27] | |
| Platelet endothelial cell adhesion molecule (PECAM-1) | The adhesion and accumulation of platelets | [28] | |
| Cadherins | E-cadherins | Cell–cell adhesion in epithelial cells | [29] |
| N-cadherins | Tumor intercellular adhesions | [30] | |
| VE-cadherins | Adhesive connections between endothelial cells | [31] | |
| Selectin | E-selectin, L-selectin, P-selectin | Adhesion of platelets to tumor cells, lymphocyte homing, endothelial cells-tumor cells, interaction of tumor cells. | [32,33,34] |
| Cell/Mice Models | Mode of Microgravity | Relevant Changes | Mechanisms | References |
|---|---|---|---|---|
| HUVECs | Progress 40P mission | 44 cell adhesion-related genes changed | / | [61] |
| EA.hy926 | SJ-10 Satellite | ICAM-1 (-), VCAM-1 (-), CD44 (+) | / | [62] |
| EA.hy926 | Random positioning machine (RPM) | Fibronectin (+), ICAM-1 (+), VCAM-1 (+) | IL-6 and IL-8 regulate adhesion molecules | [63] |
| Endothelial cells in the carotid artery of Rat | Hindlimb unweighted (HLU) | E-selectin (+), VCAM-1 (+) | / | [64] |
| Human umbilical vein endothelial cells (HUVECs) | Rotary cell culture system (RCCS) | Focal adhesions (-), actin fiber formation (-), apoptosis (+) | mTOR/Apaf-1 and miR-22 signaling pathway | [65] |
| Cell/Mice Models | Mode of Microgravity | Relevant Changes | Mechanisms | References |
|---|---|---|---|---|
| Human M1 macrophages | SpaceX CRS-3 mission | ICAM-1 (-) | Mechanically sensitive signals in the cell-polycarbonate binding region | [67] |
| Differentiated human U937 cells | 2D clinostat, SIMBOX/Shenzhou-8 mission | ICAM-1 (+) | / | [68] |
| Rat | HLU | E-selectin (+), VCAM-1 (+), MCP-1(+), recruitment of monocyte to aortic endothelium | NF-κB pathway | [69,70] |
| Peripheral monocyte | Space missions | CD26L (-), HLA-DR (-) | / | [71] |
| Peripheral monocyte | Parabolic flight | Reduced peripheral monocyte adhere to ICAM-1 | / | [72] |
| Cell Models | Mode of Microgravity | Relevant Changes | Mechanisms | References |
|---|---|---|---|---|
| BL6-10 melanoma cells | RPM | Focal adhesions (paxillin and vinculin) (-), apoptosis (+) | FAK/RhoA-regulated mTORC1 and AMPK signaling pathway | [74,75] |
| U251 cells | RPM | Focal adhesions(-), cell viability and migration (-) | FAK/RhoA/Rock and FAK/Nek2 signaling pathway | [76] |
| MCF-7 cells | RPM | Focal adhesions(-), β1 integrin(-), β4 integrin (-) | Decreased kinases activity (such as FAK, PYK2, and ILK) | [77] |
| PC-3 cells | RPM | Adhesion (-), Talin1, Vinculin, and Cdh1 in multicellular spheroids (+) | / | [78] |
| Lung cancer cells line (CRL-5889) | RPM | Adhesion (-), spherical arrangement of the actin filaments, and apoptosis (+) | / | [79] |
| MCF-7 cells | TEXUS 54 rocket mission | E-cadherin (-), β1 integrin (-), actin arrangement | / | [80] |
| MCF-7 cells | RPM | E-cadherin (-), multicellular spheroids formation | E-cadherin autodegradation pathway | [81] |
| FTC-133 cell line | TEXUS-53 Mission | ICAM-1 (+), VCAM-1 (+), cofolin1(+) | / | [82] |
| FTC-133 cell line | RPM | ICAM-1 (-), cofolin1(-), disorganized vinculin | / | [82] |
| Cell Models | Mode of Microgravity | Relevant Changes | Mechanisms | References |
|---|---|---|---|---|
| hMSCs | RPM | VCAM-1+ cells (-), VCAM-1 (-), disrupted actin cytoskeleton, vinculin redistribution | / | [85,86] |
| Rat MSCs | SJ-10 Satellite | VCAM1 (-), ICAM1 (-), CD44 (-), vinculin (-), actin filaments depolymerization, hepatogenic differentiation (+) | Upregulataion of hepatocyte-specific cytokeratin 18 and albumin | [87] |
| Rat MSCs | HLU | Vinculin-containing focal adhesions (-), osteogenesis (-) | / | [88] |
| hMSCs | Rotating wall Vessel | Autophosphorylation of FAK and PYK2 (-), osteogenesis (-) | MAPK/ERK/Runx2 pathway | [89] |
| ADSCs | RPM | CD44 (+), β1 integrin (+), cell aggregation (+) | / | [90] |
| NSCLCs | RPM | Cell adhesion (-), stemness features (-) | Decreased Nanog and Oct4 genes | [91] |
| Cell Models | Mode of Microgravity | Relevant Changes | Mechanisms | References |
|---|---|---|---|---|
| Rat osteoblasts | Space flight | Cell adhesion (-) | Decreased osteopontin | [94] |
| hFOB 1.19 cells | RPM | Cell adhesion (-) | / | [95] |
| Human primary osteoblasts | RPM | Cell adhesion (-), mesenchymal-like phenotype | Decreased β1 integrin | [96] |
| Primary osteoblasts and Osteoblastic ROS cells | Foton M3 satellite | Focal adhesions (-) | Partly ERK proliferative-dependent pathway | [93,97] |
| Osteoblastic ROS cells | Parabolic flight and clinostat | Cytoskeleton disorganization, vinculin spots disassembly | β1 integrin-mediated | [98] |
| Human MG-63 cells | Foton M3 satellite | Number of focal contacts (-) | Rho GTPase signaling pathway | [99] |
| Cell/Mice Models | Mode of Microgravity | Relevant Changes | Mechanisms | References |
|---|---|---|---|---|
| Vascular smooth muscle cells (VSMCs) | RPM | Cell adhesion (-), disrupted cytoskeleton, contractile phenotype | / | [102] |
| Rat | HLU | Focal adhesions number in a basilar artery (-) | Increased p-FAK Y397 and p-Src Y418 | [103] |
| Skeletal muscle stem/progenitor cells (SMPCs) | Clinostat rotation system | Cell adhesion (-), myotubes number (-) | TRAF6/ERK pathway | [104] |
| Cell Models | Mode of Microgravity | Relevant Changes | Mechanisms | References |
|---|---|---|---|---|
| Primary cells from human brain nervous tissue | RPM | Cell adhesion (-), apoptosis (+) | Disorganized β-tubulin structures | [106] |
| Human adult retinal pigment epithelium cells | RPM | Cell adhesion (-) | Reduced expression of β1 and β3 integrin | [107] |
| Human keratinocytes | RPM | Cell adhesion (-), E-cadherin (-) | / | [108] |
| Normal human dermal fibroblasts (NHDF) | RPM | ECM proteins Adhesion molecules Cytoskeleton | Regulate of β1 integrin and E-cadherin | [109] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Zhang, K.; Wei, D.; Tian, Y.; Gao, Y.; Chen, Z.; Qian, A. The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 3031. https://doi.org/10.3390/ijms21093031
Lin X, Zhang K, Wei D, Tian Y, Gao Y, Chen Z, Qian A. The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion. International Journal of Molecular Sciences. 2020; 21(9):3031. https://doi.org/10.3390/ijms21093031
Chicago/Turabian StyleLin, Xiao, Kewen Zhang, Daixu Wei, Ye Tian, Yongguang Gao, Zhihao Chen, and Airong Qian. 2020. "The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion" International Journal of Molecular Sciences 21, no. 9: 3031. https://doi.org/10.3390/ijms21093031