A Purified Aspartic Protease from Akkermansia Muciniphila Plays an Important Role in Degrading Muc2
Abstract
:1. Introduction
2. Results
2.1. Purification of Amuc_1434*
2.2. Amuc_1434* Activity Test and Kinetic Study
2.3. Effect of pH on the Activity of Amuc_1434*
2.4. Effect of Temperature on the Activity and Half-Life of Amuc_1434*
2.5. Effects of Inhibitors on Amuc_1434*
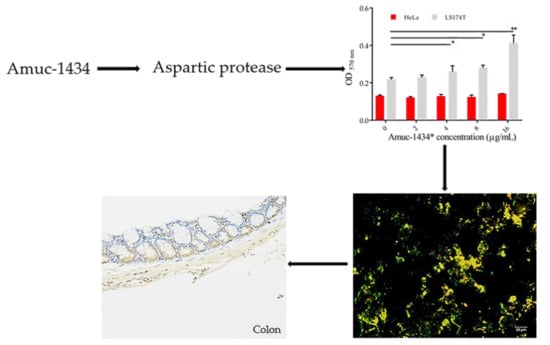
2.6. Association between Amuc_1434* and Muc2
2.7. Amuc_1434* Degradation of Muc2
2.8. Amuc_1434 Localization
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Induction and Expression of Proteins
4.3. Purification and Identification of Proteins
4.4. Western Blot
4.5. Activity Test of Purified Amuc_1434*
4.6. Kinetics of Amuc_1434* Catalyzed Reactions
4.7. Effect of pH and Temperature on Amuc_1434* Activity
4.8. Half-Life Study
4.9. Effect of Inhibitors on Proteolytic Activity
4.10. Cell Attachment Assay
4.11. Muc2 Degradation Experiment
4.12. Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. Muc2 Immunofluorescence
4.14. Immunohistochemistry
4.15. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | bicinchonininc acid |
| BSA | bovine serum albumin |
| CRC | colorectal cancer |
| DAB | diaminobenzidine |
| DIO | 3,3′-dioctadecyloxacarbocyanine perchlorate |
| DMSO | dimethyl sulfoxide |
| ECL | electrochemiluminescence |
| EDTA | ethylene Diamine Tetraacetic Acid |
| ELISA | enzyme–linked immunosorbent assay |
| EP | eppendorf |
| HRP | horseradish peroxidase |
| IPTG | isopropyl β-D-Thiogalactoside |
| MW | molecular weight |
| Mucin 2 | Muc2 |
| PBS | phosphate buffer saline |
| PE | phycoerythrin |
| PMP | pseudomyxoma peritonei |
| PMSF | phenylmethylsulphonyl fluoride |
| PAGE | polyacrylamide gel electrophoresis |
| RIPA | radio immunoprecipitation assay |
| SDS | sodium dodecylsulphate |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
References
- Andrianifahanana, M.; Moniaux, N.; Batra, S.K. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Et Biophys. Acta Rev. Cancer 2006, 1765, 189–222. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, I.; Ponnusamy, M.P.; Macha, M.A.; Haridas, D.; Majhi, P.D.; Kaur, S.; Jain, M.; Batra, S.K.; Ganti, A.K. Mucins in Lung Cancer Diagnostic, Prognostic, and Therapeutic Implications. J. Thorac. Oncol. 2015, 10, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Van Putten, J.P.M.; Strijbis, K. Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Itoh, Y.; Kamata-Sakurai, M.; Denda-Nagai, K.; Nagai, S.; Tsuiji, M.; Ishii-Schrade, K.; Okada, K.; Goto, A.; Fukayama, M.; Irimura, T. Identification and expression of human epiglycanin/MUC21: A novel transmembrane mucin. Glycobiology 2008, 18, 74–83. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, D.W.; Jang, K.T.; Lee, K.T.; Choi, S.H.; Heo, J.S.; Lee, J.K.; Paik, S.W.; Rhee, J.C. High expression of intestinal-type mucin (MUC2) in intraductal papillary mucinous neoplasms coexisting with extrapancreatic gastrointestinal cancers. Pancreas 2006, 32, 186–189. [Google Scholar] [CrossRef]
- Allen, A.; Hutton, D.A.; Pearson, J.P. The MUC2 gene product: A human intestinal mucin. Int. J. Biochem. Cell Biol. 1998, 30, 797–801. [Google Scholar] [CrossRef]
- Yu, X.W.; Rong, W.; Xu, F.L.; Xu, G.Y.; Sun, Y.R.; Feng, M.Y. Expression and clinical significance of Mucin and E-cadherin in colorectal tumors. Ai Zheng Aizheng Chin. J. Cancer 2007, 26, 1204–1210. [Google Scholar]
- Inoue, M.; Takahashi, S.; Yamashina, I.; Kaibori, M.; Okumura, T.; Kamiyama, Y.; Vichier-Guerre, S.; Cantacuzene, D.; Nakada, H. High density O-glycosylation of the MUC2 tandem repeat unit by N-acetylgalactosaminyltransferase-3 in colonic adenocarcinoma extracts. Cancer Res. 2001, 61, 950–956. [Google Scholar]
- Desseyn, J.L.; Buisine, M.P.; Porchet, N.; Aubert, J.P.; Degand, P.; Laine, A. Evolutionary history of the 11p15 human mucin gene family. J. Mol. Evol. 1998, 46, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Velcich, A.; Yang, W.C.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Buller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that Muc2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Henley, S.J.; Singh, S.D.; King, J.; Wilson, R.J.; O’Neil, M.E.; Ryerson, A.B. Invasive Cancer Incidence and Survival—United States, 2012. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Gansler, T.; Ganz, P.A.; Grant, M.; Greene, F.L.; Johnstone, P.; Mahoney, M.; Newman, L.A.; Oh, W.K.; Thomas, C.R., Jr.; Thun, M.J.; et al. Sixty Years of CA: A Cancer Journal for Clinicians. Ca-A Cancer J. Clin. 2010, 60, 345–350. [Google Scholar] [CrossRef]
- Bu, X.D.; Li, N.; Tian, X.Q.; Li, L.; Wang, J.S.; Yu, X.J.; Huang, P.L. Altered expression of MUC2 and MUC5AC in progression of colorectal carcinoma. World J. Gastroenterol. 2010, 16, 4089–4094. [Google Scholar] [CrossRef]
- Chiang, J.M.; Yeh, C.Y.; Changchien, C.R.; Chen, J.S.; Tang, R.; Chen, J.R. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int. J. Colorectal Dis. 2010, 25, 941–947. [Google Scholar] [CrossRef]
- Lungulescu, C.V.; Raileanu, S.; Afrem, G.; Ungureanu, B.S.; Florescu, D.N.; Gheonea, I.A.; Sovaila, S.; Craitoiu, S. Histochemical and immunohistochemical study of mucinous rectal carcinoma. J. Med. Life 2017, 10, 139–143. [Google Scholar]
- Tozawa, E.; Ajioka, Y.; Watanabe, H.; Nishikura, K.; Mukai, G.; Suda, T.; Kanoh, T.; Hatakeyama, K. Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: Is mucinous carcinoma a distinct histological entity? Pathol. Res. Pract. 2007, 203, 567–574. [Google Scholar] [CrossRef]
- Byrd, J.C.; Bresalier, R.S. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 77–99. [Google Scholar] [CrossRef]
- O’Connell, J.T.; Hacker, C.M.; Barsky, S.H. MUC2 is a molecular marker for pseudomyxoma peritonei. Mod. Pathol. 2002, 15, 958–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astashchanka, A.; Shroka, T.M.; Jacobsen, B.M. Mucin 2 (MUC2) modulates the aggressiveness of breast cancer. Breast Cancer Res. Treat. 2019, 173, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.S.; Khandeparkar, S.G.S.; Joshi, A.R.; Kulkarni, M.M.; Dhande, B.; Lengare, P.; Phegade, L.A.; Narkhede, K. Immunohistochemical Study of MUC1, MUC2 and MUC5AC Expression in Primary Breast Carcinoma. J. Clin. Diagn. Res. JCDR 2017, 11, EC30–EC34. [Google Scholar] [CrossRef]
- Bademler, S.; Zirtiloglu, A.; Sari, M.; Ucuncu, M.Z.; Dogru, E.B.; Karabulut, S. Clinical Significance of Serum Membrane-Bound Mucin-2 Levels in Breast Cancer. Biomolecules 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojnev, S.; Ristic-Petrovic, A.; Velickovic, L.J.; Krstic, M.; Bogdanovic, D.; Do Throng, K.; Ristic, A.; Conic, I.; Stefanovic, V. Prognostic significance of mucin expression in urothelial bladder cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 4945–4958. [Google Scholar] [PubMed]
- Yu, J.-H.; Zheng, J.-B.; Qi, J.; Yang, K.; Wu, Y.-H.; Wang, K.; Wang, C.-B.; Sun, X.-J. Bile acids promote gastric intestinal metaplasia by upregulating CDX2 and MUC2 expression via the FXR/NF-B signalling pathway. Int. J. Oncol. 2019, 54, 879–892. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; El-Bahrawy, M. Expression profile of mucins (MUC1, MUC2, MUC5AC, and MUC6) in ovarian mucinous tumours: Changes in expression from benign to malignant tumours. Histopathology 2015, 66, 529–535. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; McGuckin, M.A.; Grencis, R.K.; Thornton, D.J. Serine protease(s) secreted by the nematode Trichuris muris degrade the mucus barrier. PLoS Negl. Trop. Dis. 2012, 6, e1856. [Google Scholar] [CrossRef]
- Gibold, L.; Garenaux, E.; Dalmasso, G.; Gallucci, C.; Cia, D.; Mottet-Auselo, B.; Fais, T.; Darfeuille-Michaud, A.; Nguyen, H.T.; Barnich, N.; et al. The Vat-AIEC protease promotes crossing of the intestinal mucus layer by Crohn’s disease-associated Escherichia coli. Cell. Microbiol. 2016, 18, 617–631. [Google Scholar] [CrossRef]
- Roka, R.; Demaude, J.; Cenac, N.; Ferrier, L.; Salvador-Cartier, C.; Garcia-Villar, R.; Fioramonti, J.; Bueno, L. Colonic luminal proteases activate colonocyte proteinase-activated receptor-2 and regulate paracellular permeability in mice. Neurogastroenterol. Motil. J. Eur. Gastrointest. Motil. Soc. 2007, 19, 57–65. [Google Scholar] [CrossRef]
- Luo, Q.; Kumar, P.; Vickers, T.J.; Sheikh, A.; Lewis, W.G.; Rasko, D.A.; Sistrunk, J.; Fleckenstein, J.M. Enterotoxigenic Escherichia coli secretes a highly conserved mucin-degrading metalloprotease to effectively engage intestinal epithelial cells. Infect. Immun. 2014, 82, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belzer, C.; de Vos, W.M. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Png, C.W.; Linden, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Ottman, N.; Davids, M.; Suarez-Diez, M.; Boeren, S.; Schaap, P.J.; Martins Dos Santos, V.A.P.; Smidt, H.; Belzer, C.; de Vos, W.M. Genome-Scale Model and Omics Analysis of Metabolic Capacities of Akkermansia muciniphila Reveal a Preferential Mucin-Degrading Lifestyle. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Noh, J.R.; Chang, D.H.; Kim, Y.H.; Kim, M.H.; Lee, E.S.; Cho, S.; Ku, B.J.; Rhee, M.S.; Kim, B.C.; et al. Elucidation of Akkermansia muciniphila Probiotic Traits Driven by Mucin Depletion. Front. Microbiol. 2019, 10, 1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Passel, M.W.; Kant, R.; Zoetendal, E.G.; Plugge, C.M.; Derrien, M.; Malfatti, S.A.; Chain, P.S.; Woyke, T.; Palva, A.; de Vos, W.M.; et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS ONE 2011, 6, e16876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Wang, M.M.; Kulinich, A.; Yao, H.L.; Ma, H.Y.; Martinez, J.E.; Duan, X.C.; Chen, H.; Cai, Z.P.; Flitsch, S.L.; et al. Biochemical characterisation of the neuraminidase pool of the human gut symbiont Akkermansia muciniphila. Carbohydr. Res. 2015, 415, 60–65. [Google Scholar] [CrossRef]
- Cruz, R.; Huesgen, P.; Riley, S.P.; Wlodawer, A.; Faro, C.; Overall, C.M.; Martinez, J.J.; Simoes, I. RC1339/APRc from Rickettsia conorii is a novel aspartic protease with properties of retropepsin-like enzymes. PLoS Pathog. 2014, 10, e1004324. [Google Scholar] [CrossRef] [Green Version]
- Salehi, M.; Aghamaali, M.R.; Sajedi, R.H.; Asghari, S.M.; Jorjani, E. Purification and characterization of a milk-clotting aspartic protease from Withania coagulans fruit. Int. J. Biol. Macromol. 2017, 98, 847–854. [Google Scholar] [CrossRef]
- Correa, I.; Plunkett, T.; Vlad, A.; Mungul, A.; Candelora-Kettel, J.; Burchell, J.M.; Taylor-Papadimitriou, J.; Finn, O.J. Form and pattern of MUC1 expression on T cells activated in vivo or in vitro suggests a function in T-cell migration. Immunology 2003, 108, 32–41. [Google Scholar] [CrossRef]
- Carraway III, K.L.; Funes, M.; Workman, H.C.; Sweeney, C. Contribution of membrane mucins to tumor progression through modulation of cellular growth signaling pathways. Curr. Top. Dev. Biol. 2007, 78, 1–22. [Google Scholar] [CrossRef]
- Mall, A.S.; Chirwa, N.; Govender, D.; Lotz, Z.; Tyler, M.; Rodrigues, J.; Kahn, D.; Goldberg, P. MUC2, MUC5AC and MUC5B in the mucus of a patient with pseudomyxoma peritonei: Biochemical and immunohistochemical study. Pathol. Int. 2007, 57, 537–547. [Google Scholar] [CrossRef]
- Mu, T.; Liang, W.; Ju, Y.; Wang, Z.; Wang, Z.; Roycik, M.D.; Sang, Q.X.; Yu, D.; Xiang, H.; Fang, X. Efficient soluble expression of secreted matrix metalloproteinase 26 in Brevibacillus choshinensis. Protein Expr. Purif. 2013, 91, 125–133. [Google Scholar] [CrossRef]
- Huang, T.; Long, M.; Huo, B. Competitive Binding to Cuprous Ions of Protein and BCA in the Bicinchoninic Acid Protein Assay. Open Biomed. Eng. J. 2010, 4, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Werneck, G.; Aliakbarian, B.; Siqueira, F.; Ferreira Filho, E.X.; Perego, P.; Converti, A.; Magalhaes, P.O.; Junior, A.P. Production, purification and characterization of an aspartic protease from Aspergillus foetidus. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 109, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Z.; Wang, H.; Ren, L.; Zhang, D.; Liang, W.; Zhang, G.; Zhang, J.; Yu, D.; Fang, X. Screening, Identification, and Characterization of an Affinity Peptide Specific to MT1-MMP and its Application in Tumor Imaging. Bioconjug. Chem. 2019, 30, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Szabady, R.L.; Yanta, J.H.; Halladin, D.K.; Schofield, M.J.; Welch, R.A. TagA is a secreted protease of Vibrio cholerae that specifically cleaves mucin glycoproteins. Microbiology (Reading, England) 2011, 157, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Junmin, S.; Hong, Y.; Shuodong, W. PGE (2) induces MUC2 and MUC5AC expression in human intrahepatic biliary epithelial cells via EP4/p38MAPK activation. Ann. Hepatol. 2013, 12, 479–486. [Google Scholar]
- Chorley, B.N.; Crews, A.L.; Li, Y.; Adler, K.B.; Minnicozzi, M.; Martin, L.D. Differential Muc2 and Muc5ac secretion by stimulated guinea pig tracheal epithelial cells in vitro. Respir. Res. 2006, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, A.A.; Pardjianto, B.; Sumitro, S.B.; Kania, N.; Handono, K. Decreased expression of MUC2 due to a decrease in the expression of lectins and apoptotic defects in colitis patients. Biochem. Biophys. Rep. 2019, 19. [Google Scholar] [CrossRef]
- Antunes, L.; Frasquilho, S.; Ostaszewski, M.; Weber, J.; Longhino, L.; Antony, P.; Baumuratov, A.; Buttini, M.; Shannon, K.M.; Balling, R.; et al. Similar alpha-Synuclein staining in the colon mucosa in patients with Parkinson’s disease and controls. Mov. Disord. J. Mov. Disord. Soc. 2016, 31, 1567–1570. [Google Scholar] [CrossRef]
- Cobo, E.R.; Kissoon-Singh, V.; Moreau, F.; Chadee, K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of beta-defensin 2. Mucosal Immunol. 2015, 8, 1360–1372. [Google Scholar] [CrossRef] [Green Version]








| Compound | Concentration (mM) | Relative Activity (%) |
|---|---|---|
| None | - | 100.0 ± 9.1 |
| EDTA | 10.0 | 149.9 ± 14.4 |
| PMSF | 1.0 | 126.9 ± 2.6 |
| Pepstatin A | 0.02 | 40.15 ± 6.7 |
| E-64 | 0.01 | 88.5 ± 4.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Wang, W.; Lan, T.; Yang, W.; Yu, D.; Fang, X.; Wu, H. A Purified Aspartic Protease from Akkermansia Muciniphila Plays an Important Role in Degrading Muc2. Int. J. Mol. Sci. 2020, 21, 72. https://doi.org/10.3390/ijms21010072
Meng X, Wang W, Lan T, Yang W, Yu D, Fang X, Wu H. A Purified Aspartic Protease from Akkermansia Muciniphila Plays an Important Role in Degrading Muc2. International Journal of Molecular Sciences. 2020; 21(1):72. https://doi.org/10.3390/ijms21010072
Chicago/Turabian StyleMeng, Xin, Wencheng Wang, Tianqi Lan, Wanxin Yang, Dahai Yu, Xuexun Fang, and Hao Wu. 2020. "A Purified Aspartic Protease from Akkermansia Muciniphila Plays an Important Role in Degrading Muc2" International Journal of Molecular Sciences 21, no. 1: 72. https://doi.org/10.3390/ijms21010072