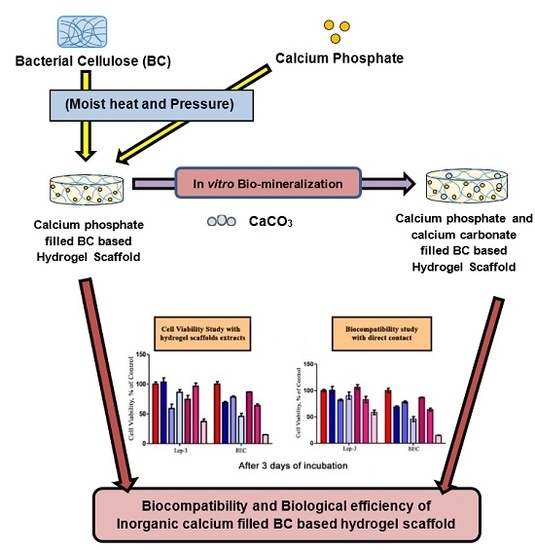
Biocompatibility and Biological Efficiency of Inorganic Calcium Filled Bacterial Cellulose Based Hydrogel Scaffolds for Bone Bioengineering
Abstract
:1. Introduction
2. Results
2.1. Cell Viability and Biocompatibility Study
2.2. Genotoxic Potential of the Studied BC Based Inorganic Calcium Filled Hydrogel Scaffolds
2.3. Study on Apoptosis/Necrosis
2.4. SEM Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Preparation of Homogenous Suspension of BC
4.3. Preparation of Inorganic Calcium Phosphate Filled BC Based Hydrogel Scaffold
4.4. Preparation of Biomineralized Inorganic Calcium Phosphate and Calcium Carbonate (CaCO3) Filled BC Based Hydrogel Scaffold
4.5. Cell cultures
4.6. Evaluation of Cell Viability and Proliferation
4.6.1. Sample Preparation
4.6.2. Indirect Experiments (IDE)
4.6.3. Direct Experiments (DE)
4.7. Study of DNA Damages
4.8. Apoptosis/Necrosis Study via Annexin V-FITC
4.9. SEM Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buenzli, P.R.; Sims, N.A. Quantifying the osteocyte network in the human skeleton. Bone 2015, 75, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kini, U.; Nandeesh, B.N. Physiology of Bone Formation, Remodeling and Metabolism, In Radionuclide and Hybrid Bone Imaging; Springer: Heidelberg, Germany, 2012; pp. 29–57. ISBN 978-3-642-02400-9. [Google Scholar]
- International Osteoporosis Foundation (IOF). 2017. Available online: https://www.iofbonehealth.org/facts-statistics (accessed on 30 August 2017).
- Castilho, M.; Dias, M.; Vorndran, E.; Gbureck, E.; Fernandes, P.; Pires, I.; Gouveia, B.; Armes, H.; Pires, E.; Rodrigues, J. Application of a 3D printed customized Implant for canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication 2014, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Deligkaris, K.; Tadele, T.S.; Olthuis, W. Hydrogel-based devices for biomedical applications. Sens. Actuators B 2010, 147, 765–774. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Biodegradation of PVP–CMC hydrogel film: A useful food packaging material. Carbohydr. Polym. 2012, 89, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.G.; De, P. Swelling properties of amino acid containing cross-linked polymeric organogels and their respective poly-electrolytic hydrogels with pH and salt responsive property. Polymer 2014, 55, 5425–5434. [Google Scholar] [CrossRef]
- Saha, N. Morphology, Absorptivity and Viscoelastic Properties of Mineralized PVP-CMC Hydrogel. AIP Conf. Proc. 2013, 1526, 292–300. [Google Scholar] [CrossRef]
- Burg, K.J.L.; Porter, S.J.F.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Gerke, R.; Götz, H.; Stein, S.; Rommens, P.M. A New Bone Substitute Developed from 3D-Prints of Polylactide (PLA) Loaded with Collagen I: An In Vitro Study. Int. J. Mol. Sci. 2017, 18, 2569. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-Y.; Kim, S.-G.; Kwon, K.-J.; Kweon, H.; Chae, W.-S.; Yang, W.-G.; Lee, E.-Y.; Seok, H. Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo. Int. J. Mol. Sci. 2017, 18, 858. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ran, J.; Yan, P.; Zheng, L.; Shen, X.; Tong, H. Rational design of a high-strength bone scaffold platform based on in situ hybridization of bacterial cellulose/nano-hydroxyapatite framework and silk fibroin reinforcing phase. J. Biomater. Sci. Polym. Ed. 2018, 29, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S. Bacterial Cellulose Production and its Industrial Applications, Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 1000150. [Google Scholar] [CrossRef]
- Shah, R.; Vyroubal, R.; Fei, H.; Saha, N.; Kitano, T.; Saha, P. Preparation of bacterial cellulose based hydrogels and their viscoelastic behavior. AIP Conf. Proc. 2015, 1662. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Kon Park, J. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martínez, R.; Artigas, N.; Gámez, B.; Rosa, J.L.; Ventura, F. Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signaling. PLoS ONE 2017, 12, e0178158. [Google Scholar] [CrossRef]
- McCleskey, E.W.; Fox, A.P.; Feldman, D.; Tsien, R.W. Different types of calcium channels. J. Exp. Biol. 1986, 124, 177–190. [Google Scholar] [PubMed]
- Dvorak-Ewell, M.M.; Chen, T.H.; Liang, N.; Garvey, C.; Liu, B.; Tu, C.; Chang, W.; Bikle, D.D.; Shoback, D.M. Osteoblast Extracellular Ca2+-Sensing Receptor Regulates Bone Development, Mineralization and Turnover. J. Bone Miner. Res. 2011, 26, 2935–2947. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, M.M.; Siddiqua, A.; Ward, D.T. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. USA 2004, 101, 5140–5145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 2–90. [Google Scholar]
- Drzewiecka, K.; Kleczewska, J.; Krasowski, M.; Łapińska, B. Mechanical properties of composite material modified with amorphous calcium phosphate. J. Achiev. Mater. Manuf. Eng. 2016, 74, 22–28. [Google Scholar] [CrossRef]
- Muthukumar, T.; Aravinthan, A.; Sharmila, J. Collagen/chitosan porous bone tissue engineering composite scaffold incorporated with Ginseng Compound, K. Carbohydr. Polym 2016, 152, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Saha, N.; Kitano, T.; Saha, P. Preparation of CaCO3-Based Biomineralized Polyvinylpyrrolidone-Carboxymethylcellulose Hydrogels and Their Viscoelastic Behaviour. J. Appl. Polym. Sci. 2014, 40237, 1–9. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Novel hydrogels of PVP–CMC and their swelling effect on viscoelastic properties. J. Appl. Polym. Sci. 2010, 117, 1703–1710. [Google Scholar] [CrossRef]
- Allou, N.B.; Yadav, A.; Pal, M.; Goswamee, R.L. Biocompatible nanocomposite of carboxymethyl cellulose and functionalized carbon–norfloxacin intercalated layered double hydroxides. Carbohydr. Polym. 2018, 186, 282–289. [Google Scholar] [CrossRef]
- Saha, N.; Shah, R.; Gupta, P.; Mandal, B.B.; Alexandrova, R.; Sikiric, M.D.; Saha, P. PVP-CMC hydrogel: An excellent bioinspired and biocompatible scaffold for osseointegration. Mater. Sci. Eng. C 2018. [Google Scholar] [CrossRef]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and characterization of bacterial cellulose/chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; de Oliveira, P.T.; Gaspar, A.M.M.; Ribeiro, S.J.L.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Pértile, R.A.N.; Moreira, S.; Gil da Costa, R.M.; Correia, A.; Guãrdao, L.; Gartner, F.; Vilanova, M.; Gama, M. Bacterial Cellulose: Long-Term Biocompatibility Studies. J. Biomater. Sci. Polym. Ed. 2018, 201223, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, M.Y.; Baek, H.-R.; Lee, K.M.; Seo, J.-H.; Lee, H.-K. Fabrication and Evaluation of Porous Beta-Tricalcium Phosphate/Hydroxyapatite (60/40) Composite as a Bone Graft Extender Using Rat Calvarial Bone Defect Model. Sci. World J. 2013, 481789, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.T.; Zakaria, Z.A.B. Biocompatibility of Bio Based Calcium Carbonate Nanocrystals Aragonite Polymorph on NIH 3T3 Fibroblast Cell Line. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Andonova-Lilova, B.; Alexandrova, R.; Rabadjieva, D.; Tepavitcharova, S. Application of cultured murine cells for initial evaluation of the biocompatibility of Mg and Zn-modified tri-calcium phosphates. C. R. Acad. Bulg. Sci. 2012, 65, 1099–1104. [Google Scholar]
- Martín, L.; Alonso, M.; Girotti, A.; Arias, F.J.; Rodriguez-Cabello, J.C. Synthesis and Characterization of Macroporous Thermosensitive Hydrogels from Recombinant Elastin-Like Polymers. Biomacromolecules 2009, 10, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Gafter, U.; Malachi, T.; Ori, Y.; Breitbart, H. The role of calcium in human lymphocyte DNA repair ability. J. Lab. Clin. Med. 1997, 130, 33–41. [Google Scholar] [CrossRef]
- Buljan, Z.I.; Ribaric, S.P.; Abram, M.; Ivankovic, A.; Spalj, S. In vitro oxidative stress induced by conventional and self-ligating brackets. Angle Orthod. 2012, 82, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadagno, N.A.; Moriconi, C.; Licursi, V.; D’Acunto, E.; Nisi, P.S.; Carucci, N.; De Jaco, A.; Cacci, E.; Negri, R.; Lupo, G.; Miranda, E. Neuroserpin polymers cause oxidative stress in a neuronal model of the dementia FENIB. Neurobiol. Dis. 2017, 103, 32–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications: A Review. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Voccoli, V.; Tonazzini, I.; Signore, G.; Caleo, M.; Cecchini, M. Role of extracellular calcium and mitochondrial oxygen species in psychosine-induced oligodendrocyte cell death. Cell Death Dis. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Review on Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Wang, Y.I. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering-Cells and Biomaterials; Daniel, E., Ed.; InTech: London, UK, 2011; pp. 569–588. ISBN 978-953-307-6638. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Saha, N.; Saha, P. Inorganic calcium filled bacterial cellulose based hydrogel scaffold: A novel biomaterial for bone tissue regeneration. Int. J. Polym. Mater. 2018, in press. [Google Scholar]
- Basu, P.; Saha, N.; Bandyopadhyay, S.; Saha, P. Rheological performance of bacterial cellulose based nonmineralized and mineralized hydrogel scaffolds. AIP Conf. Proc. 2017, 1843, 050008. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Struillou, X.; Rakic, M.; Badran, Z.; Macquigneau, L.; Colombeix, C.; Pilet, P.; Verner, C.; Gauthier, O.; Weiss, P.; Soueidan, A. The association of hydrogel and biphasic calcium phosphate in the treatment of dehiscence-type peri-implant defects: An experimental study in dogs. J. Mater. Sci. Mater. Med. 2013, 24, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Rauch, M.W.; Dressler, M.; Scheel, H.; Opdenbosch, D.V.; Zollfrank, C. Mineralization of Calcium Carbonates in Cellulose Gel Membranes. Eur. J. Inorg. Chem. 2012, 32, 5192–5198. [Google Scholar] [CrossRef]





| Sample Index | PVP (g) | CMC (g) | BC (g) | PEG (g) | Agar (g) | Glycerin (mL) | β-TCP/HA (g) | Water (mL) |
|---|---|---|---|---|---|---|---|---|
| BC-PVP | 0.5 | 0.0 | 0.5 | 1 | 2 | 1 | 0.0/0.0 | 95 |
| BC-CMC | 0.0 | 0.5 | 0.5 | 1 | 2 | 1 | 0.0/0.0 | 95 |
| BC-PVP-β-TCP/HA | 0.5 | 0.0 | 0.5 | 1 | 2 | 1 | 0.2/0.8 | 94 |
| BC-CMC-β-TCP/HA | 0.0 | 0.5 | 0.5 | 1 | 2 | 1 | 0.2/0.8 | 94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, P.; Saha, N.; Alexandrova, R.; Andonova-Lilova, B.; Georgieva, M.; Miloshev, G.; Saha, P. Biocompatibility and Biological Efficiency of Inorganic Calcium Filled Bacterial Cellulose Based Hydrogel Scaffolds for Bone Bioengineering. Int. J. Mol. Sci. 2018, 19, 3980. https://doi.org/10.3390/ijms19123980
Basu P, Saha N, Alexandrova R, Andonova-Lilova B, Georgieva M, Miloshev G, Saha P. Biocompatibility and Biological Efficiency of Inorganic Calcium Filled Bacterial Cellulose Based Hydrogel Scaffolds for Bone Bioengineering. International Journal of Molecular Sciences. 2018; 19(12):3980. https://doi.org/10.3390/ijms19123980
Chicago/Turabian StyleBasu, Probal, Nabanita Saha, Radostina Alexandrova, Boyka Andonova-Lilova, Milena Georgieva, George Miloshev, and Petr Saha. 2018. "Biocompatibility and Biological Efficiency of Inorganic Calcium Filled Bacterial Cellulose Based Hydrogel Scaffolds for Bone Bioengineering" International Journal of Molecular Sciences 19, no. 12: 3980. https://doi.org/10.3390/ijms19123980