Calcium Signaling in Vertebrate Development and Its Role in Disease
Abstract
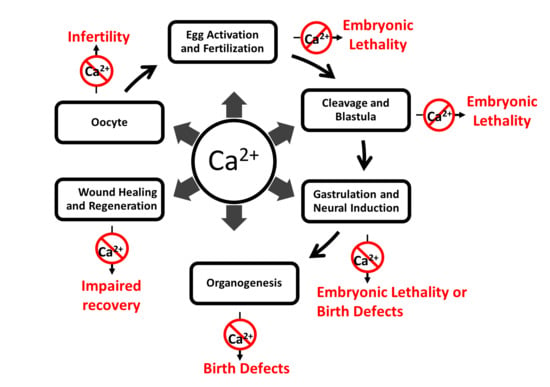
:1. Introduction
2. Calcium Activity during Development and Its Role in Disease
2.1. Fertilization and Egg Activation
2.2. Cleavage and Blastula Stages
2.3. Gastrulation
2.4. Neural Induction
2.5. Organogenesis
2.5.1. Nervous System Development
Neural Tube Closure
Neurotransmitter Phenotype Specification
2.5.2. Muscle Development
2.5.3. Heart Development
2.5.4. Kidney Development
2.5.5. Immune System
3. Wound Healing and Regeneration
3.1. Wound Healing before Formation of Immune System
3.2. Wound Healing Following the Formation of Immune System
3.3. Regeneration
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Database GeneCards. GeneCards—Human Genes|Gene Database|Gene Search. Available online: https://www.genecards.org/ (accessed on 23 September 2018).
- Lew, V.L.; Tsien, R.Y.; Miner, C.; Bookchin, R.M. Physiological [Ca2+]Ilevel and Pump-Leak Turnover in Intact Red Cells Measured Using an Incorporated Ca Chelator. Nature 1982, 298, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I. Intracellular Calcium. In Signal Transduction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 381–439. [Google Scholar] [CrossRef]
- Suzuki, M.; Sato, M.; Koyama, H.; Hara, Y.; Hayashi, K.; Yasue, N.; Imamura, H.; Fujimori, T.; Nagai, T.; Campbell, R.E.; et al. Distinct Intracellular Ca2+ Dynamics Regulate Apical Constriction and Differentially Contribute to Neural Tube Closure. Development 2017, 144, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R.; Bielmeier, C.; Harz, H.; Bates, J.; Stadler, C.; Hildebrand, A.; Classen, A.K. Calcium Spikes, Waves and Oscillations in a Large, Patterned Epithelial Tissue. Sci. Rep. 2017, 7, 42786. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Kanemaru, K.; Ishii, K.; Ohkura, M.; Okubo, Y.; Iino, M. Imaging Intraorganellar Ca2+ at Subcellular Resolution Using CEPIA. Nat. Commun. 2014, 5, 4153. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The Versatility and Universality of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Dupont, G.; Combettes, L.; Leybaert, L. Calcium Dynamics: Spatio-Temporal Organization from the Subcellular to the Organ Level. Int. Rev. Cytol. 2007, 261, 193–245. [Google Scholar] [PubMed]
- Markova, O.; Sénatore, S.; Chardès, C.; Lenne, P.F. Calcium Spikes in Epithelium: Study on Drosophila Early Embryos. Sci. Rep. 2015, 5, 11379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Olson, E.C.; Spitzer, N.C. Spontaneous Neuronal Calcium Spikes and Waves during Early Differentiation. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 6325–6335. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. National Center for Advancing Translational Sciences (NCATS). Available online: https://ncats.nih.gov/ (accessed on 23 September 2018).
- Zhaurova, K. Genetic Causes of Adult-Onset Disorders. Nat. Educ. 2008, 1, 49. [Google Scholar]
- Robertson, S.; Lin, R. Oocyte-to-Zygote Transition. Semin. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Brosens, J.J.; Quenby, S.; Takeda, S. (Eds.) Treatment Strategy for Unexplained Infertility and Recurrent Miscarriage; Springer: Midtown Manhattan, NY, USA, 2018. [Google Scholar]
- Kashir, J.; Deguchi, R.; Jones, C.; Coward, K.; Stricker, S.A. Comparative Biology of Sperm Factors and Fertilization-Induced Calcium Signals across the Animal Kingdom. Mol. Reprod. Dev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.L.; Mayfield, B.L.; Duray, A.M.; Tembo, M.; Beleny, D.O.; Napolitano, M.A.; Sauer, M.L.; Wisner, B.W.; Carlson, A.E. Extracellular Ca2+ Is Required for Fertilization in the African Clawed Frog, Xenopus Laevis. PLoS ONE 2017, 12, e0170405. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Sassa, T.; Higashijima, S.; Okamoto, H.; Miyawaki, A. Transgenic Zebrafish for Ratiometric Imaging of Cytosolic and Mitochondrial Ca2+ response in Teleost Embryo. Cell Calcium 2013, 54, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, K.S.; Whittingham, D.G.; Cobbold, P.H. Free Ca2+ Increases in Exponential Phases during Mouse Oocyte Activation. Nature 1981, 294, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, R.; Shirakawa, H.; Oda, S.; Mohri, T.; Miyazaki, S. Spatiotemporal Analysis of Ca(2+) Waves in Relation to the Sperm Entry Site and Animal-Vegetal Axis during Ca(2+) Oscillations in Fertilized Mouse Eggs. Dev. Biol. 2000, 218, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Buitrago, M.; Bonte, D.; De Sutter, P.; Leybaert, L.; Heindryckx, B. Single Ca2+ Transients vs. Oscillatory Ca2+ Signaling for Assisted Oocyte Activation: Limitations and Benefits. Reproduction 2018, 155, R105–R119. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-L.; Stein, P.; Jefferson, W.N.; Padilla-Banks, E.; Williams, C.J. Calcium Influx-Mediated Signaling Is Required for Complete Mouse Egg Activation. Proc. Natl. Acad. Sci. USA 2012, 109, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.L.; Williams, C.J. Calcium Signaling in Mammalian Egg Activation and Embryo Development: The Influence of Subcellular Localization. Mol. Reprod. Dev. 2012, 79, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, M.L.; Zhang, Y.; Erxleben, C.F.; Padilla-Banks, E.; McDonough, C.E.; Miao, Y.-L.; Armstrong, D.L.; Williams, C.J. CaV3.2 T-Type Channels Mediate Ca2+ Entry during Oocyte Maturation and Following Fertilization. J. Cell Sci. 2015, 128, 4442–4452. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.S.; Dong, J.B.; Huang, X.Y.; Sun, F.Z.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. Ca(2+) Oscillations Induced by a Cytosolic Sperm Protein Factor Are Mediated by a Maternal Machinery That Functions only Once in Mammalian Eggs. Development 2000, 127, 1141–1150. [Google Scholar] [PubMed]
- Saunders, C.M. Ca2+ Oscillations Triggered by Sperm PLCz. 2002. [Google Scholar]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Ferrer Buitrago, M.; Ardestani, G.; Bassett, A.; Fox, S.; Navarrete, F.; de Sutter, P.; et al. PLCζ Is the Physiological Trigger of the Ca2+ Oscillations That Induce Embryogenesis in Mammals but Conception Can Occur in Its Absence. Development 2017, 144, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wang, C.; Machaty, Z. STIM1 Is Required for Ca2+ Signaling during Mammalian Fertilization. Dev. Biol. 2012, 367, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kuroda, K.; Tanaka, A.; Watanabe, S. Fertilization Failure. In Treatment Strategy for Unexplained Infertility and Recurrent Miscarriage; Springer: Singapore, 2018; pp. 7–17. [Google Scholar]
- Ferrer-Buitrago, M.; Dhaenens, L.; Lu, Y.; Bonte, D.; Vanden Meerschaut, F.; De Sutter, P.; Leybaert, L.; Heindryckx, B. Human Oocyte Calcium Analysis Predicts the Response to Assisted Oocyte Activation in Patients Experiencing Fertilization Failure after ICSI. Hum. Reprod. 2018, 33, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J.L.; Ukoumunne, O.C.; Baker, H.W.G.; Breheny, S.; Jaques, A.M.; Garrett, C.; Healy, D.; Amor, D. Increased Risk of Blastogenesis Birth Defects, Arising in the First 4 Weeks of Pregnancy, after Assisted Reproductive Technologies. Hum. Reprod. 2010, 25, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jin, J.; Lee, G.A.; Silva, E.; Donoghue, M. Cross-Species Functional Analyses Reveal Shared and Separate Roles for Sox11 in Frog Primary Neurogenesis and Mouse Cortical Neuronal Differentiation. Biol. Open 2016, 5, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Chen, Y.; Hung, T.S.; Miller, A.L.; Shipley, A.M.; Webb, S.E. Inhibition of SOCE Disrupts Cytokinesis in Zebrafish Embryos via Inhibition of Cleavage Furrow Deepening. Int. J. Dev. Biol. 2015, 59, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Aw, J.T.M.; Webb, S.E.; Miller, A.L. SOCE Proteins, STIM1 and Orai1, Are Localized to the Cleavage Furrow during Cytokinesis of the First and Second Cell Division Cycles in Zebrafish Embryos. Zygote 2016, 24, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Eno, C.; Gomez, T.; Slusarski, D.C.; Pelegri, F. Slow Calcium Waves Mediate Furrow Microtubule Reorganization and Germ Plasm Compaction in the Early Zebrafish Embryo. Development 2018, 145, dev156604. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, L.; Bruchas, M.R.; Solnica-Krezel, L. Imaging Early Embryonic Calcium Activity with GCaMP6s Transgenic Zebrafish. Dev. Biol. 2017, 430, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.E.; Miller, A.L. Ca2+ Signaling during Vertebrate Somitogenesis. Acta Pharmacol. Sin. 2006, 27, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.H.; Webb, S.E.; Chan, C.M.; Zhang, J.; Miller, A.L. Establishment of a Transitory Dorsal-Biased Window of Localized Ca2+ Signaling in the Superficial Epithelium Following the Mid-Blastula Transition in Zebrafish Embryos. Dev. Biol. 2009, 327, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Webb, S.E.; Daguzan, C.; Moreau, M.; Miller, A.L. Imaging Patterns of Calcium Transients during Neural Induction in Xenopus Laevis Embryos. J. Cell Sci. 2000, 113, 3519–3529. [Google Scholar] [PubMed]
- Hara, Y.; Nagayama, K.; Yamamoto, T.S.; Matsumoto, T.; Suzuki, M.; Ueno, N. Directional Migration of Leading-Edge Mesoderm Generates Physical Forces: Implication in Xenopus Notochord Formation during Gastrulation. Dev. Biol. 2013, 382, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Brivanlou, A.H. Proposal of a Model of Mammalian Neural Induction. Dev. Biol. 2007, 308, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Shindo, A.; Hara, Y.; Yamamoto, T.S.; Ohkura, M.; Nakai, J.; Ueno, N. Tissue-Tissue Interaction-Triggered Calcium Elevation Is Required for Cell Polarization during Xenopus Gastrulation. PLoS ONE 2010, 5, e8897. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamamoto, T.S.; Ueno, N. Intracellular Calcium Signal at the Leading Edge Regulates Mesodermal Sheet Migration during Xenopus Gastrulation. Sci. Rep. 2018, 8, 2433. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Vaquer, A.; Hadjantonakis, A.K. Birth Defects Associated with Perturbations in Preimplantation, Gastrulation, and Axis Extension: From Conjoined Twinning to Caudal Dysgenesis. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Gilland, E.; Miller, A.L.; Karplus, E.; Baker, R.; Webb, S.E. Imaging of Multicellular Large-Scale Rhythmic Calcium Waves during Zebrafish Gastrulation. Proc. Natl. Acad. Sci. USA 1999, 96, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.E.; Moreau, M.; Leclerc, C.; Miller, A.L. Calcium Transients and Neural Induction in Vertebrates. Cell Calcium 2005, 37, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.Y.F.; Webb, S.E.; Chan, C.M.; Thisse, B.; Thisse, C.; Miller, A.L. Characterization of Ca2+ signaling in the External Yolk Syncytial Layer during the Late Blastula and Early Gastrula Periods of Zebrafish Development. Biochim. Biophys. Acta 2013, 1833, 1641–1656. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Neant, I.; Webb, S.E.; Miller, A.L.; Leclerc, C. Calcium Signalling during Neural Induction in Xenopus Laevis Embryos. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Néant, I.; Moreau, M. Early Neural Development in Vertebrates Is also a Matter of Calcium. Biochimie 2011, 93, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Tang, Y.; Davila, J.; Deng, S.; Chen, L.; Miller, E.; Wernig, M.; Graef, I.A. Calcineurin Signaling Regulates Neural Induction through Antagonizing the BMP Pathway. Neuron 2014, 82, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, M.; Néant, I.; Webb, S.E.; Miller, A.L.; Riou, J.F.; Leclerc, C. Ca2+ coding and Decoding Strategies for the Specification of Neural and Renal Precursor Cells during Development. Cell Calcium 2016, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Creton, R. The Calcium Pump of the Endoplasmic Reticulum Plays a Role in Midline Signaling during Early Zebrafish Development. Dev. Brain Res. 2004, 151, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.E.; Miller, A.L. Ca2+ signaling and Early Embryonic Patterning during the Blastula and Gastrula Periods of Zebrafish and Xenopus Development. Biochim. Biophys. Acta 2006, 1763, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, J.B.; Ewald, A.J.; Harland, R.M.; Fraser, S.E. Calcium Signaling during Convergent Extension in Xenopus. Curr. Biol. 2001, 11, 652–661. [Google Scholar] [CrossRef]
- Lange, L.; Marks, M.; Liu, J.; Wittler, L.; Bauer, H.; Piehl, S.; Bläß, G.; Timmermann, B.; Herrmann, B.G. Patterning and Gastrulation Defects Caused by the Tw18 Lethal Are Due to Loss of Ppp2r1a. Biol. Open 2017, 6, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, Y.; Taira, M. The Molecular Basis of the Gastrula Organizer in Amphibians and Cnidarians. In Reproductive and Developmental Strategies. Diversity and Commonality in Animals; Springer: Tokyo, Japan, 2018; pp. 667–708. [Google Scholar]
- Barth, L.G.; Barth, L.J. Sequential Induction of the Presumptive Epidermis of the Rana Pipiens Gastrula. Biol. Bull. 1964, 127, 413–427. [Google Scholar] [CrossRef]
- Stableford, L.T. A Study of Calcium in the Early Development of the Amphibian Embryo. Dev. Biol. 1967, 16, 303–314. [Google Scholar] [CrossRef]
- Leclerc, C.; Néant, I.; Moreau, M. The Calcium: An Early Signal That Initiates the Formation of the Nervous System during Embryogenesis. Front. Mol. Neurosci. 2012, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-K.; Lee, H.-S.; Moody, S.A. Neural Transcription Factors: From Embryos to Neural Stem Cells. Mol. Cells 2014, 37, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M.; Sonnen, J.A.; Keene, C.D.; Hevner, R.F.; Montine, T.J. Molecular Basis of Diseases of the Nervous System, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Wilde, J.J.; Petersen, J.R.; Niswander, L. Genetic, Epigenetic, and Environmental Contributions to Neural Tube Closure. Annu. Rev. Genet. 2014, 48, 583–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.E.; Copp, A.J. Neural Tube Closure: Cellular, Molecular and Biomechanical Mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Smedley, M.J.; Stanisstreet, M. Calcium and Neurulation in Mammalian Embryos II. Effects of Cytoskeletal Inhibitors and Calcium Antagonists on the Neural Folds of Rat Embryos. Development 1986, 93, 167–178. [Google Scholar]
- Christodoulou, N.; Skourides, P.A.A. Cell-Autonomous Ca2+ Flashes Elicit Pulsed Contractions of an Apical Actin Network to Drive Apical Constriction during Neural Tube Closure. Cell Rep. 2015, 13, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wolf, F.; Großhans, J. In Vivo Optochemical Control of Cell Contractility at Single Cell Resolution by Ca2+ Induced Myosin Activation. bioRxiv 2018. [Google Scholar] [CrossRef]
- Sahu, S.U.; Visetsouk, M.R.; Garde, R.J.; Hennes, L.; Kwas, C.; Gutzman, J.H. Calcium Signals Drive Cell Shape Changes during Zebrafish Midbrain–hindbrain Boundary Formation. Mol. Biol. Cell 2017, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wajid, S.; Morales-Diaz, H.; Khairallah, S.M.; Smith, W.C. T-Type Calcium Channel Regulation of Neural Tube Closure and EphrinA/EPHA Expression. Cell Rep. 2015, 13, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Sequerra, E.B.; Goyal, R.; Castro, P.A.; Levin, J.B.; Borodinsky, L.N. NMDA Receptor Signaling Is Important for Neural Tube Formation and for Preventing Antiepileptic Drug-Induced Neural Tube Defects NMDA Receptor Signaling Is Important for Neural Tube Formation and for Preventing Antiepileptic Drug-Induced Neural Tube Defec. J. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Borodinsky, L.N. Xenopus Laevis as a Model Organism for the Study of Spinal Cord Formation, Development, Function and Regeneration. Front. Neural Circuits 2017, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.E.; Mai, C.T.; Canfield, M.A.; Rickard, R.; Wang, Y.; Meyer, R.E.; Anderson, P.; Mason, C.A.; Collins, J.S.; Kirby, R.S.; et al. Updated National Birth Prevalence Estimates for Selected Birth Defects in the United States, 2004–2006. Birth Defects Res. Part A—Clin. Mol. Teratol. 2010, 88, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Borodinsky, L.N.; Belgacem, Y.H. Crosstalk among Electrical Activity, Trophic Factors and Morphogenetic Proteins in the Regulation of Neurotransmitter Phenotype Specification. J. Chem. Neuroanat. 2016, 73, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, N.C. Neurotransmitter Switching? No Surprise. Neuron 2015, 86, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, N.C.; Debaca, R.C.; Allen, K.A.; Holliday, J. Calcium Dependence of Differentiation of GABA Immunoreactivity in Spinal Neurons. J. Comp. Neurol. 1993, 337, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.B.; Miller, L.E.; Herbst, W.A.; Saha, M.S. The Role of Voltage-Gated Calcium Channels in Neurotransmitter Phenotype Specification: Coexpression and Functional Analysis in Xenopus Laevis. J. Comp. Neurol. 2014, 522, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Borodinsky, L.N.; Root, C.M.; Cronin, J.A.; Sann, S.B.; Gu, X.; Spitzer, N.C. Activity-Dependent Homeostatic Specification of Transmitter Expression in Embryonic Neurons. Nature 2004, 429, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.W.; Kurtz, L.M.; Spitzer, N.C. CJun Integrates Calcium Activity and Tlx3 Expression to Regulate Neurotransmitter Specification. Nat. Neurosci. 2010, 13, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.B.; Valakh, V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 2015, 87, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Serrano, J.L.; Paluszkiewicz, S.M.; Martin, B.S.; Kaufmann, W.E.; Corbin, J.G.; Huntsman, M.M. Defective GABAergic Neurotransmission and Pharmacological Rescue of Neuronal Hyperexcitability in the Amygdala in a Mouse Model of Fragile X. Syndrome. J. Neurosci. 2010, 30, 9929–9938. [Google Scholar] [CrossRef] [PubMed]
- Cellot, G.; Cherubini, E. GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. Front. Pediatr. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H.; Treves, S.; Zorzato, F.; Sarkozy, A.; Ochala, J.; Sewry, C.; Phadke, R.; Gautel, M.; Muntoni, F. Congenital Myopathies: Disorders of Excitation-Contraction Coupling and Muscle Contraction. Nat. Rev. Neurol. 2018, 14, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Suryakumar, G.; Rathor, R. Role of Defective Ca2+ signaling in Skeletal Muscle Weakness: Pharmacological Implications. J. Cell Commun. Signal. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, E.A.G.; Hilfer, S.R. Calcium Dependence and Contraction in Somite Formation. Tissue Cell 1982, 14, 435–449. [Google Scholar] [CrossRef]
- Ferrari, M.B.; Spitzer, N.C. Calcium Signaling in the Developing Xenopus Myotome. Dev. Biol. 1999, 213, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Webb, S.E.; Love, D.R.; Miller, A.L. Visualization, Characterization and Modulation of Calcium Signaling during the Development of Slow Muscle Cells in Intact Zebrafish Embryos. Int. J. Dev. Biol. 2011, 55, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Kelu, J.J.; Webb, S.E.; Parrington, J.; Galione, A.; Miller, A.L. Ca2+ release via Two-Pore Channel Type 2 (TPC2) Is Required for Slow Muscle Cell Myofibrillogenesis and Myotomal Patterning in Intact Zebrafish Embryos. Dev. Biol. 2017, 425, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Kelu, J.J.; Chan, H.L.H.; Webb, S.E.; Cheng, A.H.H.; Ruas, M.; Parrington, J.; Galione, A.; Miller, A.L. Two-Pore Channel 2 Activity Is Required for Slow Muscle Cell-Generated Ca2+ Signaling during Myogenesis in Intact Zebrafish. Int. J. Dev. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.B.; Rohrbough, J.; Spitzer, N.C. Spontaneous Calcium Transients Regulate Myofibrillogenesis in Embryonic Xenopus Myocytes. Dev. Biol. 1996, 178, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.R.; Podugu, S.P.; Ferrari, M.B. Spatiotemporal Characterization of Short versus Long Duration Calcium Transients in Embryonic Muscle and Their Role in Myofibrillogenesis. Dev. Biol. 2006, 292, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Zvaritch, E.; Depreux, F.; Kraeva, N.; Loy, R.E.; Goonasekera, S.A.; Boncompagni, S.; Kraev, A.; Gramolini, A.O.; Dirksen, R.T.; Franzini-Armstrong, C.; et al. An Ryr1I4895T Mutation Abolishes Ca2+ Release Channel Function and Delays Development in Homozygous Offspring of a Mutant Mouse Line. Proc. Natl. Acad. Sci. USA 2007, 104, 18537–18542. [Google Scholar] [CrossRef] [PubMed]
- Farini, A.; Sitzia, C.; Cassinelli, L.; Colleoni, F.; Parolini, D.; Giovanella, U.; Maciotta, S.; Colombo, A.; Meregalli, M.; Torrente, Y. Inositol 1,4,5-Trisphosphate (IP3)-Dependent Ca2+ Signaling Mediates Delayed Myogenesis in Duchenne Muscular Dystrophy Fetal Muscle. Development 2016, 143, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Allard, B. From Excitation to Intracellular Ca2+ movements in Skeletal Muscle: Basic Aspects and Related Clinical Disorders. Neuromuscul. Disord. 2018. [Google Scholar] [CrossRef] [PubMed]
- Stiber, J.A.; Rosenberg, P.B. The Role of Store-Operated Calcium Influx in Skeletal Muscle Signaling. Cell Calcium 2011, 49, 341–349. [Google Scholar] [CrossRef] [PubMed]
- McCarl, C.A.; Picard, C.; Khalil, S.; Kawasaki, T.; Röther, J.; Papolos, A.; Kutok, J.; Hivroz, C.; LeDeist, F.; Plogmann, K.; et al. ORAI1 Deficiency and Lack of Store-Operated Ca2+ entry Cause Immunodeficiency, Myopathy, and Ectodermal Dysplasia. J. Allergy Clin. Immunol. 2009, 124, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; McCarl, C.-A.; Papolos, A.; Khalil, S.; Lüthy, K.; Hivroz, C.; LeDeist, F.; Rieux-Laucat, F.; Rechavi, G.; Rao, A.; et al. STIM1 Mutation Associated with a Syndrome of Immunodeficiency and Autoimmunity. N. Engl. J. Med. 2009, 360, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Pisaniello, A. The Block of Ryanodine Receptors Selectively Inhibits Fetal Myoblast Differentiation. J. Cell Sci. 2003. [Google Scholar] [CrossRef]
- Webb, S.E.; Cheung, C.C.Y.; Chan, C.M.; Love, D.R.; Miller, A.L. Application of Complementary Luminescent and Fluorescent Imaging Techniques to Visualize Nuclear and Cytoplasmic Ca2+ Signalling during the In Vivo Differentiation of Slow Muscle Cells in Zebrafish Embryos under Normal and Dystrophic Conditions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kloesel, B.; Dinardo, J.A.; Body, S.C. Cardiac Embryology and Molecular Mechanisms of Congenital Heart Disease: A Primer for Anesthesiologists. Anesthesia Analgesia 2016. [Google Scholar] [CrossRef] [PubMed]
- Schleich, J.M.; Abdulla, T.; Summers, R.; Houyel, L. An Overview of Cardiac Morphogenesis. Arch. Cardiovasc. Dis. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorman, A.; Webb, S.; Brown, N.; Lamers, W.; Anderson, R. Development of the Heart: Formation of the Cardiac Chambers and Arterial Trunks. Heart 2003, 89, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Créton, R.; Speksnijder, J.E.; Jaffe, L.F. Patterns of Free Calcium in Zebrafish Embryos. J. Cell Sci. 1998, 111, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Tallini, Y.N.; Ohkura, M.; Choi, B.-R.; Ji, G.; Imoto, K.; Doran, R.; Lee, J.; Plan, P.; Wilson, J.; Xin, H.-B.; et al. Imaging Cellular Signals in the Heart in Vivo: Cardiac Expression of the High-Signal Ca2+ Indicator GCaMP2. Proc. Natl. Acad. Sci. USA 2006, 103, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; Makuck, R.F.; Rivkees, S.A. Intracellular Calcium Plays an Essential Role in Cardiac Development. Dev. Dyn. 2003, 227, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, A.; Feridooni, T.; Zhang, F.; Pasumarthi, K.B.S. The Effects of Calcium Channel Blockade on Proliferation and Differentiation of Cardiac Progenitor Cells. Cell Calcium 2014, 55, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.E.; Miller, A.L. Calcium Signalling during Embryonic Development. Nat. Rev. Mol. Cell Biol. 2003, 4, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.P. Patterning the Vertebrate Heart. Nat. Rev. Genet. 2002, 3, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.J.; Vize, P.D. Synergism between Pax-8 and Lim-1 in Embryonic Kidney Development. Dev. Biol. 1999, 214, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Buisson, I.; Le Bouffant, R.; Futel, M.; Riou, J.F.; Umbhauer, M. Pax8 and Pax2 Are Specifically Required at Different Steps of Xenopus Pronephros Development. Dev. Biol. 2015, 397, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Tena, J.J.; Neto, A.; de la Calle-Mustienes, E.; Bras-Pereira, C.; Casares, F.; Gómez-Skarmeta, J.L. Odd-Skipped Genes Encode Repressors That Control Kidney Development. Dev. Biol. 2007, 301, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Webb, S.E.; Miller, A.L.; Moreau, M. An Increase in Intracellular Ca2+ is Involved in Pronephric Tubule Differentiation in the Amphibian Xenopus Laevis. Dev. Biol. 2008, 321, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.R.; Hidaka, S.; Gretz, N.; Witzgall, R. Molecular Basis of Autosomal-Dominant Polycystic Kidney Disease. Cell. Mol. Life Sci. 2002, 59, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Boucek, M.M.; Snyderman, R. Calcium Influx Requirement for Human Neutrophil Chemotaxis: Inhibition by Lanthanum Chloride. Science 1976, 193, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Oh-hora, M.; Rao, A. Calcium Signaling in Lymphocytes. Curr. Opin. Immunol. 2008, 20, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Oh-Hora, M. Calcium Signaling in the Development and Function of T-Lineage Cells. Immunol. Rev. 2009. [Google Scholar] [CrossRef] [PubMed]
- Beerman, R.W.W.; Matty, M.A.A.; Au, G.G.G.; Looger, L.L.L.; Choudhury, K.R.R.; Keller, P.J.J.; Tobin, D.M.M. Direct In Vivo Manipulation and Imaging of Calcium Transients in Neutrophils Identify a Critical Role for Leading-Edge Calcium Flux. Cell Rep. 2015, 13, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.W.; Maxfield, F.R. Transient Increases in Cytosolic Free Calcium Appear to Be Required for the Migration of Adherent Human Neutrophils. J. Cell Biol. 1990, 110, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Lewis, R.S.; Rao, A. Molecular Basis of Calcium Signaling in Lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010, 28, 491–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, Y.; Nishida, K.; Fujii, Y.; Hirano, T.; Hikida, M.; Kurosaki, T. Essential Function for the Calcium Sensor STIM1 in Mast Cell Activation and Anaphylactic Responses. Nat. Immunol. 2008, 9, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; DeHaven, W.I.; Bird, G.S.; Billingsley, J.M.; Wang, H.; Rao, P.E.; Hutchings, A.B.; Jouvin, M.-H.; Putney, J.W.; Kinet, J.-P. Defective Mast Cell Effector Functions in Mice Lacking the CRACM1 Pore Subunit of Store-Operated Calcium Release–activated Calcium Channels. Nat. Immunol. 2008, 9, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Degen, K.E.; Gourdie, R.G. Embryonic Wound Healing: A Primer for Engineering Novel Therapies for Tissue Repair. Birth Defects Res. Part C—Embryo Today Rev. 2012, 96, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, S.; Volk, S.W.; Bayat, A. Regenerative Healing, Scar-Free Healing and Scar Formation across the Species: Current Concepts and Future Perspectives. Exp. Dermatol. 2014, 23, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Herrgen, L.; Voss, O.P.; Akerman, C.J. Calcium-Dependent Neuroepithelial Contractions Expel Damaged Cells from the Developing Brain. Dev. Cell 2014, 31, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.V.; Jacinto, A. The Role of Transcription-Independent Damage Signals in the Initiation of Epithelial Wound Healing. Nat. Rev. Mol. Cell Biol. 2013, 14, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Razzell, W.; Evans, I.R.; Martin, P.; Wood, W. Calcium Flashes Orchestrate the Wound Inflammatory Response through Duox Activation and Hydrogen Peroxide Release. Curr. Biol. 2013, 23, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A Tissue-Scale Gradient of Hydrogen Peroxide Mediates Rapid Wound Detection in Zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.K.; Borodinsky, L.N. Spontaneous Calcium Transients Manifest in the Regenerating Muscle and Are Necessary for Skeletal Muscle Replenishment. Cell Calcium 2014, 56, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J. Platelets in Wound Healing and Regenerative Medicine. Platelets 2018, 15, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Braun, A.; Haining, E.J.; Tseng, Y.-L.; Kraft, P.; Schuhmann, M.K.; Gotru, S.K.; Chen, W.; Hermanns, H.M.; Stoll, G.; et al. Partially Defective Store Operated Calcium Entry and Hem(ITAM) Signaling in Platelets of Serotonin Transporter Deficient Mice. PLoS ONE 2016, 11, e0147664. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Thielmann, I.; Gupta, S.; Subramanian, H.; Stegner, D.; van Kruchten, R.; Dietrich, A.; Gambaryan, S.; Heemskerk, J.W.M.; Hermanns, H.M.; et al. Orai1-Induced Store-Operated Ca 2+ Entry Enhances Phospholipase Activity and Modulates Canonical Transient Receptor Potential Channel 6 Function in Murine Platelets. J. Thromb. Haemost. 2014, 12, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Gotru, S.K.; Chen, W.; Kraft, P.; Becker, I.C.; Wolf, K.; Stritt, S.; Zierler, S.; Hermanns, H.M.; Rao, D.; Perraud, A.L.; et al. TRPM7 Kinase Controls Calcium Responses in Arterial Thrombosis and Stroke in Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lysaght, M.J.; Jaklenec, A.; Deweerd, E. Great Expectations: Private Sector Activity in Tissue Engineering, Regenerative Medicine, and Stem Cell Therapeutics. Tissue Eng. Part A 2008, 14, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.A.; Juarez, M.T.; Hermann, A.; Sasik, R.; Hardiman, G.; McGinnis, W. Serine Proteolytic Pathway Activation Reveals an Expanded Ensemble of Wound Response Genes in Drosophila. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Tsujioka, H.; Kunieda, T.; Katou, Y.; Shirahige, K.; Kubo, T. Unique Gene Expression Profile of the Proliferating Xenopus Tadpole Tail Blastema Cells Deciphered by RNA-Sequencing Analysis. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Mouse Gene Expression Data Search. Available online: http://www.informatics.jax.org/gxd (accessed on 25 September 2018).
- ZFIN Expression Search. Available online: https://zfin.org/action/expression/search (accessed on 25 September 2018).
- Gene Expression Search. Available online: http://www.xenbase.org/geneExpression/geneExpressionSearch.do?method=display (accessed on 25 September 2018).
- Kolios, G.; Moodley, Y. Introduction to Stem Cells and Regenerative Medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.; Daks, A.; Fedorova, O.; Shuvalov, O.; Barlev, N.A. Ca2+-Depended Signaling Pathways Regulate Self-Renewal and Pluripotency of Stem Cells. Cell Biol. Int. 2018, 42, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Mao, J.J. Stem Cell-Based Composite Tissue Constructs for Regenerative Medicine. Biotechnol. Bioeng. 2005, 91, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A. Tissue Engineering for Artificial Organs: Regenerative Medicine, Smart Diagnostics and Personalized Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 1–2. [Google Scholar]
- Jergova, S.; Gajavelli, S.; Varghese, M.S.; Shekane, P.; Sagen, J. Analgesic Effect of Recombinant GABAergic Cells in a Model of Peripheral Neuropathic Pain. Cell Transplant. 2016, 25, 629–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méry, A.; Aimond, F.; Ménard, C.; Mikoshiba, K.; Michalak, M.; Pucéat, M. Initiation of Embryonic Cardiac Pacemaker Activity by Inositol 1,4,5-Trisphosphate-Dependent Calcium Signaling. Mol. Biol. Cell 2005, 16, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Limpitikul, W.B.; Dick, I.E.; Tester, D.J.; Boczek, N.J.; Limphong, P.; Yang, W.; Choi, M.H.; Babich, J.; Disilvestre, D.; Kanter, R.J.; et al. A Precision Medicine Approach to the Rescue of Function on Malignant Calmodulinopathic Long-QT Syndrome. Circ. Res. 2017, 120, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, S.; Sindelar, R.; Saha, M. Calcium Signaling in Vertebrate Development and Its Role in Disease. Int. J. Mol. Sci. 2018, 19, 3390. https://doi.org/10.3390/ijms19113390
Paudel S, Sindelar R, Saha M. Calcium Signaling in Vertebrate Development and Its Role in Disease. International Journal of Molecular Sciences. 2018; 19(11):3390. https://doi.org/10.3390/ijms19113390
Chicago/Turabian StylePaudel, Sudip, Regan Sindelar, and Margaret Saha. 2018. "Calcium Signaling in Vertebrate Development and Its Role in Disease" International Journal of Molecular Sciences 19, no. 11: 3390. https://doi.org/10.3390/ijms19113390