TBC1D21 Potentially Interacts with and Regulates Rap1 during Murine Spermatogenesis
Abstract
:1. Introduction
1.1. Small Guanosine Triphosphate-Binding Proteins and GTPase-Activating Proteins
1.2. Identification and Characterization of a Novel GTPase-Activating Protein: TBC1D21
1.3. Rap1 Function in Male Reproduction
2. Results
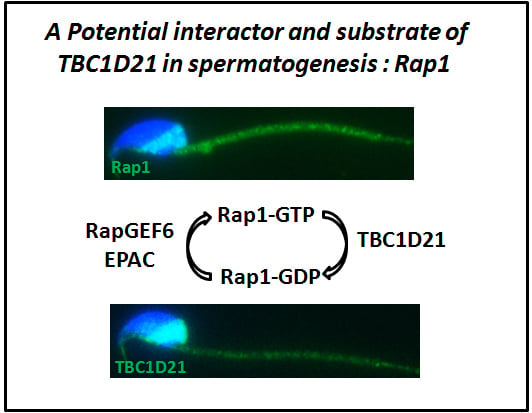
2.1. TBC1D21 Interacts with Rap1 in NTERA-2 cl.D1 Cells
2.2. Expression Patterns of Rap1 during Murine Spermatogenesis
2.3. Dynamic Localization of Rap1 and TBC1D21 during Murine Spermiogenesis
2.4. Rap1 Is Regulated by TBC1D21
3. Discussion
3.1. TBC1D21 Regulates Rabs and Other Small GTPases
3.2. Molecular and Cellular Functions of Rap1 during Mammalian Spermatogenesis
3.3. GTPase-Activating Proteins and Other Small GTPases during Mammalian Spermiogenesis
3.4. TBC1D21 and Rap1 in Male Infertility
4. Materials and Methods
4.1. Liquid Chromatography–Tandem Mass Spectrometry, Co-Immunoprecipitation, and Immunoblotting
4.2. Real-Time Polymerase Chain Reaction
4.3. Rap1 Pull-Down Assay
4.4. Isolated Testicular Germ Cells and Immunofluorescence Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Eathiraj, S.; Munson, M.; Lambright, D.G. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 2006, 442, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Perez-Sala, D.; Gajate, C.; Jimenez, B.; Rodriguez, P.; Lacal, J.C. Localization of rap1 and rap2 proteins in the gelatinase-containing granules of human neutrophils. FEBS Lett. 1993, 326, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Lucking, R.; Lawrey, J.D.; Gillevet, P.M.; Sikaroodi, M.; Dal-Forno, M.; Berger, S.A. Multiple ITS haplotypes in the genome of the lichenized basidiomycete Cora inversa (Hygrophoraceae): Fact or artifact? J. Mol. Evol. 2014, 78, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Nieves, E.; Che, F.Y.; Wang, J.; Jin, L.; Murray, J.W.; Gordon, K.; Angeletti, R.H.; Wolkoff, A.W. Proteomic analysis of endocytic vesicles: Rab1a regulates motility of early endocytic vesicles. J. Cell Sci. 2011, 124, 765–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.L.; Wu, H.; Tang, B.H.; Qiu, S.; Li, Z.L. Atmospheric corrections of passive microwave data for estimating land surface temperature. Opt. Express 2013, 21, 15654–15663. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.M.; Teng, Y.N.; Hsieh, T.Y.; Lin, Y.S.; Kuo, P.L. Identification of ten novel genes involved in human spermatogenesis by microarray analysis of testicular tissue. Fertil. Steril. 2006, 86, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.M.; Kuo, Y.C.; Wang, Y.Y.; Kuo, P.L. Identification and characterization of a novel Rab GTPase-activating protein in spermatids. Int. J. Androl. 2011, 34, e358–e367. [Google Scholar] [CrossRef] [PubMed]
- Boersema, P.J.; Raijmakers, R.; Lemeer, S.; Mohammed, S.; Heck, A.J. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 2009, 4, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Lin, C.M.; Huang, C.J.; Chen, S.K.; Wu, S.T.; Chiang, H.S.; Ku, W.C. Dual Roles of 17-beta Estradiol in Estrogen Receptor-dependent Growth Inhibition in Renal Cell Carcinoma. Cancer Genom. Proteom. 2016, 13, 219–230. [Google Scholar]
- Mori-Quiroz, L.M.; Shimkin, K.W.; Rezazadeh, S.; Kozlowski, R.A.; Watson, D.A. Copper-Catalyzed Amidation of Primary and Secondary Alkyl Boronic Esters. Chemistry 2016, 22, 15654–15658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H.; Liu, M.; Dong, B. Reprogrammable Logic Gate and Logic Circuit Based on Multistimuli-Responsive Raspberry-like Micromotors. ACS Appl. Mater. Interfaces 2016, 8, 15654–15660. [Google Scholar] [CrossRef] [PubMed]
- Pizzochero, M.; Bonfanti, M.; Martinazzo, R. Hydrogen on silicene: Like or unlike graphene? Phys. Chem. Chem. Phys. 2016, 18, 15654–15666. [Google Scholar] [CrossRef] [PubMed]
- Aivatiadou, E.; Mattei, E.; Ceriani, M.; Tilia, L.; Berruti, G. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant. Mol. Biol. Cell 2007, 18, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Holcomb, C.; Moore, H.P. Expression and localization of two low molecular weight GTP-binding proteins, Rab8 and Rab10, by epitope tag. Proc. Natl. Acad. Sci. USA 1993, 90, 6508–6512. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Kim, M.; Kingsbury, T.J.; Civin, C.I.; Cheng, W.C. Regulation of RAB5C is important for the growth inhibitory effects of MiR-509 in human precursor-B acute lymphoblastic leukemia. PLoS ONE 2014, 9, e111777. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, H.; Sugimoto, Y.; Matsuzaki, T.; Ikawa, Y.; Noda, M. A ras-related gene with transformation suppressor activity. Cell 1989, 56, 77–84. [Google Scholar] [CrossRef]
- Yang, Z.W.; Wreford, N.G.; de Kretser, D.M. A quantitative study of spermatogenesis in the developing rat testis. Biol. Reprod. 1990, 43, 629–635. [Google Scholar] [PubMed]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Quiroz, J.A.; Wolkoff, A.W. Rab1a regulates sorting of early endocytic vesicles. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G412–G424. [Google Scholar] [CrossRef] [PubMed]
- Frasa, M.A.; Koessmeier, K.T.; Ahmadian, M.R.; Braga, V.M. Illuminating the functional and structural repertoire of human TBC/RABGAPs. Nat. Rev. Mol. Cell Biol. 2012, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2009, 15, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.B.; Zhang, L.; Chen, D.F.; Yang, F.; Yang, J.S.; Yang, W.J. Two p90 ribosomal S6 kinase isoforms are involved in the regulation of mitotic and meiotic arrest in Artemia. J. Biol. Chem. 2014, 289, 16006–16015. [Google Scholar] [CrossRef] [PubMed]
- Wolgemuth, D.J.; Laurion, E.; Lele, K.M. Regulation of the mitotic and meiotic cell cycles in the male germ line. Recent Prog. Horm. Res. 2002, 57, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Grallert, B.; Sipiczki, M. Common genes and pathways in the regulation of the mitotic and meiotic cell cycles of Schizosaccharomyces pombe. Curr. Genet. 1991, 20, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Larance, M.; Ramm, G.; Stockli, J.; van Dam, E.M.; Winata, S.; Wasinger, V.; Simpson, F.; Graham, M.; Junutula, J.R.; Guilhaus, M.; et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 2005, 280, 37803–37813. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Qin, G.; Jungemann, C.; Hu, M. Strain-modulated electronic and thermal transport properties of two-dimensional O-silica. Nanotechnology 2016, 27, 265706. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.S.; Rahman, M.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Increased male fertility using fertility-related biomarkers. Sci. Rep. 2015, 5, 15654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, C.E.; Yang, Z.; Rimer, R.; Park-Min, K.H.; Macaubas, C.; Mellins, E.D.; Novack, D.V.; Faccio, R. Tmem178 acts in a novel negative feedback loop targeting NFATc1 to regulate bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 15654–15659. [Google Scholar] [CrossRef] [PubMed]
- Martinolich, A.J.; Neilson, J.R. Pyrite formation via kinetic intermediates through low-temperature solid-state metathesis. J. Am. Chem. Soc. 2014, 136, 15654–15659. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Farnochi, M.C.; Chulze, S.N.; Ramirez, M.L. Two-dimensional environmental profiles of growth and fumonisin production by Fusarium proliferatum on a wheat-based substrate. Int. J. Food Microbiol. 2014, 182–183, 9–17. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn O’Brien, K.L.; Varghese, A.C.; Agarwal, A. The genetic causes of male factor infertility: A review. Fertil. Steril. 2010, 93, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Raicu, F.; Gatta, V.; Zuccarello, D.; Palka, G.; Foresta, C. Male infertility: Role of genetic background. Reprod. Biomed. Online 2007, 14, 734–745. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, Y.M.; Wang, Y.Y.; Yu, I.S.; Lin, Y.W.; Wang, Y.H.; Wu, C.M.; Pan, H.A.; Chao, S.C.; Yen, P.H.; et al. The expression level of septin12 is critical for spermiogenesis. Am. J. Pathol. 2009, 174, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Yang, V.C.; Huang, S.C.; Lo, N.W. Stage-dependent expression of extra-embryonic tissue-spermatogenesis-homeobox gene 1 (ESX1) protein, a candidate marker for X chromosome-bearing sperm. Reprod. Fertil. Dev. 2005, 17, 447–455. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, C.-C.; Lin, Y.-H.; Wang, Y.-Y.; Wu, Y.-Y.; Chen, M.-F.; Ku, W.-C.; Chiang, H.-S.; Lai, T.-H. TBC1D21 Potentially Interacts with and Regulates Rap1 during Murine Spermatogenesis. Int. J. Mol. Sci. 2018, 19, 3292. https://doi.org/10.3390/ijms19113292
Ke C-C, Lin Y-H, Wang Y-Y, Wu Y-Y, Chen M-F, Ku W-C, Chiang H-S, Lai T-H. TBC1D21 Potentially Interacts with and Regulates Rap1 during Murine Spermatogenesis. International Journal of Molecular Sciences. 2018; 19(11):3292. https://doi.org/10.3390/ijms19113292
Chicago/Turabian StyleKe, Chih-Chun, Ying-Hung Lin, Ya-Yun Wang, Ying-Yu Wu, Mei-Feng Chen, Wei-Chi Ku, Han-Sun Chiang, and Tsung-Hsuan Lai. 2018. "TBC1D21 Potentially Interacts with and Regulates Rap1 during Murine Spermatogenesis" International Journal of Molecular Sciences 19, no. 11: 3292. https://doi.org/10.3390/ijms19113292