Zerumbone, a Tropical Ginger Sesquiterpene of Zingiber officinale Roscoe, Attenuates α-MSH-Induced Melanogenesis in B16F10 Cells
Abstract
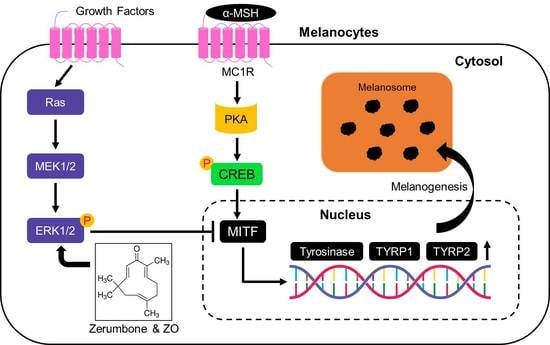
:1. Introduction
2. Results
2.1. Zerumbone (ZER) Suppresses α-MSH Induced Melanogenesis in B16F10 Mouse Melanoma Cells
2.2. Zerumbone Suppresses Gene Expression of Melanogenesis Transcription Factor, MITF, and Its Target Genes in G361 Human Melanoma Cells
2.3. Zerumbone Suppresses Melanogenic Genes and Enzymes Expression in Mouse B16F10 Cells
2.4. Anti-Melanogenic Effect of Zerumbone Is through Phosphorylation of ERK1/2
2.5. Anti-Melanogenic Effect of Zingiber Officinale (ZO) Extracts
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Cell Viability Assay
4.3. Immunoblotting and Immunoprecipitation
4.4. Measurement of Intracellular and Extracellular Melanin Content
4.5. Quantitative RT-PCR
4.6. Cellular Tyrosinase Activity Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MSH | Alpha-melanocyte stimulating hormone |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| MITF | Microphthalmia-associated transcription factor |
| TYRP1 | Tyrosinase-related protein 1 |
| TYRP2 | Tyrosinase-related protein 2 |
| CREB | cAMP response element binding protein |
| PKA | Protein Kinase A |
| ERK1/2 | Extracellular signal-regulated kinase ½ |
References
- Miyamura, Y.; Coelho, S.G.; Wolber, R.; Miller, S.A.; Wakamatsu, K.; Zmudzka, B.Z.; Ito, S.; Smuda, C.; Passeron, T.; Choi, W.; et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, P.A. Melanogenesis and melanoma. Pigment Cell Res. 2003, 16, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Sriwiriyanont, P.; Kobayashi, T.; Nagasawa, A.; Yoshida, H.; Ohuchi, A.; Kitahara, T.; Visscher, M.O.; Takema, Y.; Tsuboi, R. Stem cell factor-KIT signaling plays a pivotal role in regulating pigmentation in mammalian hair. J. Pathol. 2009, 218, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin suppresses alpha-msh-induced melanogenesis in b16f10 mouse melanoma cells by inhibiting tyrosinase activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003, 116, 1699–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Hemesath, T.J.; Takemoto, C.M.; Horstmann, M.A.; Wells, A.G.; Price, E.R.; Fisher, D.Z.; Fisher, D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000, 14, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Choi, B.R.; Lee, E.K.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Lee, Y.J.; Ku, S.K. Inhibitory effect of dried pomegranate concentration powder on melanogenesis in B16F10 melanoma cells; involvement of p38 and PKA signaling pathways. Int. J. Mol. Sci. 2015, 16, 24219–24242. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Han, M.; Yao, C.; Chung, J.H. Chaetocin inhibits IBMX-induced melanogenesis in B16F10 mouse melanoma cells through activation of ERK. Chem. Biol. Interact. 2016, 245, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Miwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillerbrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; Yeap, S.K.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Abdul, A.B.; How, C.W. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. BioMed Res. Int. 2014, 2014, 920742. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Lee, C.L.; Korivi, M.; Liao, J.W.; Rajendran, P.; Wu, J.J.; Hseu, Y.C. Zerumbone protects human skin keratinocytes against UVA-irradiated damages through Nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, W.; Yuan, X.; Li, D.; Gu, W.; Gao, T. Endothelin-1 enhances the melanogenesis via MITF-GPNMB pathway. BMB Rep. 2013, 46, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G.; Yada, Y.; Kimura, M. Signaliing mechanisms of endothelin-induced mitogenesis and melanogenesis in human melanocytes. Biochem. J. 1996, 314, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yonezawa, T.; Teruya, T.; Woo, J.T.; Cha, B.Y. Nobiletin, a polymethoxy flavonoid, reduced endothelin-1 plus SCF-induced pigmentation in human melanocytes. Photochem. Photobiol. 2015, 91, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gong, L.; Hadda, M.M.; Bischof, O.; Campisi, J.; Yeh, E.H.; Medrano, E.E. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp. Cell Res. 2000, 255, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wellbrock, C.; Rana, S.; Paterson, H.; Pickersgill, H.; Brummelkamp, T.; Marais, R. Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS ONE 2008, 3, e2734. [Google Scholar] [CrossRef] [PubMed]
- Scherle, P.A.; Jones, E.A.; Favata, M.F.; Daulerio, A.J.; Convington, M.B.; Nurnberg, S.A.; Magolda, R.L.; Trzaskos, J.M. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J. Immunol. 1998, 161, 5681–5686. [Google Scholar] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, A.A.; et al. Plants of the genus Zingiber as a source of bioactive phytochemicals: from tradition to pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Singh, V.; Ali, M. Chemical composition and antimicrobial activity of fresh rhizome essential oil of Zingiber Officinale Roscoe. Pharmacogn. J. 2016, 8, 185–190. [Google Scholar] [CrossRef]
- Nam, J.H.; Nam, D.Y.; Lee, D.U. Valencene from the Rhizomes of Cyperus rotundus inhibits skin photoaging-related ion channels and UV-induced melanogenesis in b16f10 melanoma cells. J. Nat. Prod. 2016, 79, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Su, C.C.; Peng, H.Y.; Chou, S.T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol inhibits α-MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. BioMed Res. Int. 2014, 2014, 842569. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Czyz, M. MITF in melanoma: Mechanisms behind its expression and activity. Cell. Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Verastegui, C.; Bille, K.; Ortonne, J.P.; Ballotti, R. Regulation of the microphthalmia-associated transcription factor gene by the Waardenburg syndrome Type 4 gene, SOX10. J. Biol. Chem. 2000, 275, 30757–30760. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Yasumoto, K.I.; Takeda, K.; Takahashi, K.; Fukuzaki, A.; Orikasa, S.; Shibahara, S. Melanocyte-specific microphthalmia-associated transcription factor isoform activates its own gene promoter through physical interaction with Lymphoid-enhancing Factor 1. J. Biol. Chem. 2002, 277, 28787–28794. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Jantan, I.; Harikrishnan, H. Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through MyD88-dependent NF-κB/MAPK/PI3K-Akt signaling pathways. Int. Immunopharmacol. 2018, 55, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.Y.; Tzeng, T.F.; Liou, S.S.; Liu, I.M. The ethanol extract of Zingiber zerumbet rhizomes mitigates vascular lesions in the diabetic retina. Vasc. Pharmacol. 2016, 76, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, S.H.; Ryu, S.R.; Lee, P.; Moon, C. Enhancing the effects of Zerumbone on THP-1 cell activation. Korean J. Clin. Lab. Sci. 2017, 49, 1–7. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Drira, R.; Sakamoto, K. Isosakuranetin, a 4′-O-methylated flavonoid, stimulates melanogenesis in B16BL6 murine melanoma cells. Life Sci. 2015, 143, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, Q.; Liu, Y.; Qiao, H.; Liu, Y. Zerumbone, a South Asian ginger sesquiterpene, induced apoptosis of pancreatic carcinoma cells through p53 signaling pathway. Evid. Based Complement. Altern. Med. 2012, 2012, 936030. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, A.; Ahuja, N.; Mercado, A.L.; Diagaradjane, P.; Raju, U.; Patel, N.; Mohindra, P.; Diep, N.; Guha, S.; Krishnan, S. Zerumbone increases oxidative stress in a thiol-dependent ROS-independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med. 2015, 4, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev. 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Lin, D.P.; Wu, C.Y.; Teng, M.C.; Sun, C.Y.; Tsai, Y.T.; Su, K.C.; Wang, S.R.; Chang, H.H. Dietary zerumbone prevents mouse corena from UVB-induced photokeratitis through inhibition of NF-κB, iNOS, and TNF-α expression and reduction of MDA accumulation. Mol. Vis. 2011, 17, 854–863. [Google Scholar] [PubMed]
- Romero-Graillet, C.; Aberdam, E.; Clement, M.; Ortonne, J.P.; Ballotti, R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J. Clin. Invest. 1997, 99, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, M.W.; Igarashi, S.; Sasaki, M.; Wakamatsu, K.; Ito, S.; Horikoshi, T. Effects of melanogenesis-inducing nitric oxide and histamine on the production of eumelanin and pheomelanin in cultured human melanocytes. Pigment Cell Res. 2003, 16, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.R.; Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Tasrip, N.A.; Mokhtar, F.; Zakaria, Z.A.; Lajis, N.H.; Israf, D.A. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia 2010, 81, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Kader, G.; Nikkon, F.; Rashid, M.A.; Yeasmin, T. Antimicrobial activities of the rhizome extract of Zingiber zerumbet Linn. Asian Pac. J. Trop. Biomed. 2011, 1, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Habsah, M.; Amran, M.; Mackeen, M.M.; Lajis, N.H.; Kikuzaki, H.; Nakatani, N.; Rahman, A.A.; Ali, A.M. Screening of Zingiberaceae extracts for antimicrobial and antioxidant activities. J. Ethnopharmacol. 2000, 72, 403–410. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S. Anti-allergic activity of some selected plants in the Zingiberaceae family. J. Ethnopharmacol. 2007, 109, 535–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.-I.; Jung, H.-J.; Lee, Y.-M.; Lee, S.; Kim, G.-H.; Kan, S.-Y.; Kang, H.; Oh, T.; Ko, H.M.; Kwak, K.-C.; et al. Zerumbone, a Tropical Ginger Sesquiterpene of Zingiber officinale Roscoe, Attenuates α-MSH-Induced Melanogenesis in B16F10 Cells. Int. J. Mol. Sci. 2018, 19, 3149. https://doi.org/10.3390/ijms19103149
Oh T-I, Jung H-J, Lee Y-M, Lee S, Kim G-H, Kan S-Y, Kang H, Oh T, Ko HM, Kwak K-C, et al. Zerumbone, a Tropical Ginger Sesquiterpene of Zingiber officinale Roscoe, Attenuates α-MSH-Induced Melanogenesis in B16F10 Cells. International Journal of Molecular Sciences. 2018; 19(10):3149. https://doi.org/10.3390/ijms19103149
Chicago/Turabian StyleOh, Taek-In, Hye-Jeong Jung, Yoon-Mi Lee, Sujin Lee, Geon-Hee Kim, Sang-Yeon Kan, Hyeji Kang, Taerim Oh, Hyun Myung Ko, Keun-Chang Kwak, and et al. 2018. "Zerumbone, a Tropical Ginger Sesquiterpene of Zingiber officinale Roscoe, Attenuates α-MSH-Induced Melanogenesis in B16F10 Cells" International Journal of Molecular Sciences 19, no. 10: 3149. https://doi.org/10.3390/ijms19103149