Triterpene Acid (3-O-p-Coumaroyltormentic Acid) Isolated From Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein
Abstract
:1. Introduction
2. Results
2.1. Isolation of the CSC Inhibitor From Aronia
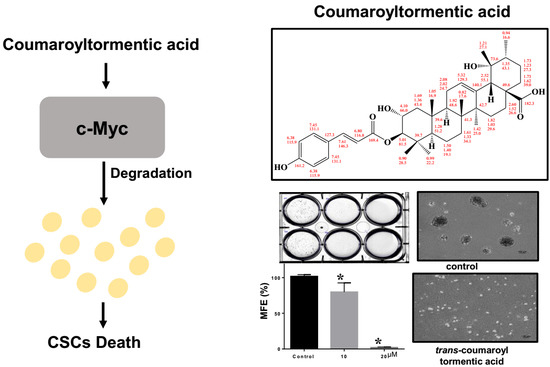
2.2. Structure Determination of the Isolated CSC Inhibitor
2.3. 3-O-p-Coumaroyltormentic Acid Inhibits Breast Cancer Cell Proliferation and Mammosphere Formation
2.4. Treatment with 3-O-trans-p-Coumaroyltormentic Acid Decreased the CD44high/CD24low-Expressing Subpopulation and the Proportion of ALDH-Positive Breast Cancer Cells
2.5. Effect of 3-O-Trans-p-Coumaroyltormentic Acid on the Protein Expression Levels of c-Myc in Breast CSCs
2.6. c-Myc is a Survival Factor for Breast CSC Formation
2.7. 3-O-trans-p-Coumaroyltormentic Acid Inhibits the Expression of Self-Renewal Genes in CSCs and the Proliferation of Mammospheres
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Structure Analysis of Purified Sample
4.5. Culture of Human Breast Cancer Cells and the Formation of Mammospheres
4.6. Automated Counting of Mammospheres
4.7. Cell Proliferation Assay
4.8. Flow Cytometric Analysis of CD44 and CD24 Expression
4.9. Clonogenic Assay
4.10. Scratch Migration Assay
4.11. Real-Time RT-PCR
4.12. ALDEFLUOR Assay
4.13. Western Blotting
4.14. Small Interfering RNA (siRNA)
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CSCs | Cancer stem cells |
| AMJ | Aronia melanocarpa juice |
| NICE | NIST’s integrated colony enumerator |
| ESI | Electrospray ionization |
| NMR | Nuclear magnetic resonance |
| COSY | Correlation spectroscopy |
| HMQC | Heteronuclear multiple-quantum correlation spectroscopy |
| HMBC | Heteronuclear multiple-bond correlation spectroscopy |
| ROI | Regions of interest |
| MFE | Mammosphere formation efficiency |
| Trans | trans-Coumaroyltormentic acid |
References
- Sic Zlabur, J.; Dobricevic, N.; Pliestic, S.; Galic, A.; Bilic, D.P.; Voca, S. Antioxidant potential of fruit juice with added chokeberry powder (aronia melanocarpa). Molecules 2017, 22, 2158. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Kang, S.H.; Moon, K.H.; Lee, J.H.; Kim, D.G.; Kim, W.; Kim, J.S.; Ahn, B.Y.; Jin, J.S. The effect of aronia berry on type 1 diabetes in vivo and in vitro. J. Med. Food 2018, 21, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find Exp. Clin. Pharmacol. 2007, 29, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Becker, M.W.; Wicha, M.; Weissman, I.; Clarke, M.F. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 2004, 14, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dontu, G.; Wicha, M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnenberg, V.S.; Donnenberg, A.D. Multiple drug resistance in cancer revisited: The cancer stem cell hypothesis. J. Clin. Pharmacol. 2005, 45, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Hwang, E.S. Quality characteristics and antioxidant activity of yogurt supplemented with aronia (aronia melanocarpa) juice. Prev. Nutr. Food Sci. 2016, 21, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Thani, N.A.; Sallis, B.; Nuttall, R.; Schubert, F.R.; Ahsan, M.; Davies, D.; Purewal, S.; Cooper, A.; Rooprai, H.K. Induction of apoptosis and reduction of MMP gene expression in the U373 cell line by polyphenolics in Aronia melanocarpa and by curcumin. Oncol. Rep. 2012, 28, 1435–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, T.; Alhosin, M.; Auger, C.; Minker, C.; Kim, J.H.; Etienne-Selloum, N.; Bories, P.; Gronemeyer, H.; Lobstein, A.; Bronner, C.; et al. Aronia melanocarpa juice induces a redox-sensitive p73-related caspase 3-dependent apoptosis in human leukemia cells. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Li, Z.; Wu, Q.; Lathia, J.D.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. c-Myc is required for maintenance of glioma cancer stem cells. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.D.; Hwang, E.S. Bioactive compound contents and antioxidant activity in aronia (aronia melanocarpa) leaves collected at different growth stages. Prev. Nutr. Food Sci. 2014, 19, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Shobharani, P.; Nanishankar, V.H.; Halami, P.M.; Sachindra, N.M. Antioxidant and anticoagulant activity of polyphenol and polysaccharides from fermented Sargassum sp. Int. J. Biol. Macromol. 2014, 65, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Hussain, S.A.; Yang, J.; Ijaz, M.U.; Liu, Q.; Suleria, H.A.R.; Song, Y. Antioxidants potential of the filamentous fungi (mucor circinelloides). Nutrients 2017, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Akazawa, H.; Tabata, K.; Manosroi, A.; Manosroi, J.; Suzuki, T.; Akihisa, T. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem. Pharm. Bull. 2011, 59, 378–381. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Q.; Yang, L.; Zhang, J.Y.; Ma, J.N.; Ma, C.M. Chemical constituents of gold-red apple and their alpha-glucosidase inhibitory activities. J. Food Sci. 2014, 79, C1970–C1983. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Nakamichi, N.; Sekino, H.; Nakano, S. Comparison of the effects of acarbose and voglibose in healthy subjects. Clin. Ther. 1997, 19, 720–729. [Google Scholar] [CrossRef]
- Geng, S.Q.; Alexandrou, A.T.; Li, J.J. Breast cancer stem cells: Multiple capacities in tumor metastasis. Cancer Lett. 2014, 349, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, D.; Semenza, G.L. In vitro assays of breast cancer stem cells. Methods Mol. Biol. 2018, 1742, 237–246. [Google Scholar] [PubMed]
- Meyer, N.; Penn, L.Z. Reflecting on 25 years with MYC. Nat. Rev. Cancer 2008, 8, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.; Cunningham, M.; Arnold, H.; Chasse, D.; Monteith, T.; Ivaldi, G.; Hahn, W.C.; Stukenberg, P.T.; Shenolikar, S.; Uchida, T.; et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell. Biol. 2004, 6, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Murakami, Y.; Tajima, A.; Matsufuji, S. Novel ubiquitin-independent nucleolar c-Myc degradation pathway mediated by antizyme 2. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Qin, S.; Schulte, B.A.; Ethier, S.P.; Tew, K.D.; Wang, G.Y. MYC inhibition depletes cancer stem-like cells in triple-negative breast cancer. Cancer Res. 2017, 77, 6641–6650. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Zhang, Y.; Zhou, J.; Zhi, Q.; Wu, M.Y.; Gong, F.R.; Shen, M.; Liu, L.; Tao, M.; Shen, B.; et al. Ginsenoside Rg3 targets cancer stem cells and tumor angiogenesis to inhibit colorectal cancer progression in vivo. Int. J. Oncol. 2018, 52, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Tharmarajah, L.; Samarakoon, S.R.; Ediriweera, M.K.; Piyathilaka, P.; Tennekoon, K.H.; Senathilake, K.S.; Rajagopalan, U.; Galhena, P.B.; Thabrew, I. In vitro anticancer effect of gedunin on human teratocarcinomal (ntera-2) cancer stem-like cells. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.M.; Shen, C.J.; Chang, C.C.; Chou, C.Y.; Tsai, C.C.; Hsu, Y.C. Inducement of apoptosis by cucurbitacin E, a tetracyclic triterpenes, through death receptor 5 in human cervical cancer cell lines. Cell Death Discov. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.H.; Mahdzir, M.A.; Din, M.F.; Awang, K.; Tanaka, Y.; Kulkeaw, K.; Ishitani, T.; Sugiyama, D. Induction of intrinsic apoptosis in leukaemia stem cells and in vivo zebrafish model by betulonic acid isolated from Walsura pinnata Hassk (Meliaceae). Phytomedicine 2017, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Cevatemre, B.; Botta, B.; Mori, M.; Berardozzi, S.; Ingallina, C.; Ulukaya, E. The plant-derived triterpenoid tingenin B is a potent anticancer agent due to its cytotoxic activity on cancer stem cells of breast cancer in vitro. Chem. Biol. Interact. 2016, 260, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lacaille-Dubois, M.A.; Wagner, H. New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine 2017. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Kim, K.A.; Yoo, G.; Kim, S.Y.; Shin, J.M.; Kim, J.H.; Jung, S.H.; Kim, J.; Nho, C.W. Phenethyl isothiocyanate suppresses cancer stem cell properties in vitro and in a xenograft model. Phytomedicine 2017, 30, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, S.; Ren, K.; Quan, M.; Song, Z.; Zou, H.; Li, D.; Cao, J. Reversal of liver cancer-associated stellate cell-induced stem-like characteristics in SMMC-7721 cells by 8-bromo-7-methoxychrysin via inhibiting STAT3 activation. Oncol. Rep. 2016, 35, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Li, W.; Wu, R.; Guo, Y.; Cheng, D.; Yang, Y.; Androulakis, I.P.; Kong, A.N. Pharmacokinetics and pharmacodynamics of the triterpenoid ursolic acid in regulating the antioxidant, anti-inflammatory, and epigenetic gene responses in rat leukocytes. Mol. Pharm. 2017, 14, 3709–3717. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, J.M.; Rao, C.V. Triterpenoids for cancer prevention and treatment: Current status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, D.A.; Chung, H.; Park, I.H.; Kim, B.H.; Oh, E.S.; Kang, D.H. Screening of breast cancer stem cell inhibitors using a protein kinase inhibitor library. Cancer Cell Int. 2017, 17. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Deng, H.-Y.; Yun, B.-S.; Lee, D.-S. Triterpene Acid (3-O-p-Coumaroyltormentic Acid) Isolated From Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein. Int. J. Mol. Sci. 2018, 19, 2528. https://doi.org/10.3390/ijms19092528
Choi HS, Kim S-L, Kim J-H, Deng H-Y, Yun B-S, Lee D-S. Triterpene Acid (3-O-p-Coumaroyltormentic Acid) Isolated From Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein. International Journal of Molecular Sciences. 2018; 19(9):2528. https://doi.org/10.3390/ijms19092528
Chicago/Turabian StyleChoi, Hack Sun, Su-Lim Kim, Ji-Hyang Kim, Hong-Yuan Deng, Bong-Sik Yun, and Dong-Sun Lee. 2018. "Triterpene Acid (3-O-p-Coumaroyltormentic Acid) Isolated From Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein" International Journal of Molecular Sciences 19, no. 9: 2528. https://doi.org/10.3390/ijms19092528