Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target
Abstract
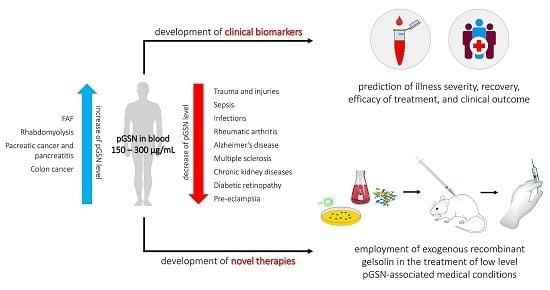
:1. Introduction
Multifunctional Role of Gelsolin in Health and Disease
2. Alterations in Plasma Gelsolin Concentrations in Different Clinical Conditions
2.1. Trauma
2.1.1. Release of Actin from Damaged Tissues as a Major Pathological Event in Trauma Patients
2.1.2. Decreased Concentrations of pGSN in Trauma Patients
2.1.3. Repletion of Physiological Level of pGSN as a Therapeutic Option in Trauma Subjects
2.2. Sepsis
2.2.1. Prognostic Value of pGSN in Sepsis Patients When Combined with Other Sepsis Biomarkers
2.2.2. Beneficial Gelsolin-Mediated Mechanisms in Animal Models of Sepsis
2.3. Infections and Infection-Associated Diseases
2.3.1. Advantageous Effect of Gelsolin on Host Anti-Infection Protection Mechanisms
2.3.2. Varied Concentrations of pGSN in Bacterial, Protozoa and Viral-Induced Diseases
2.3.3. The Clinical Potential of Application of Plasma Gelsolin as Mucolytic Agent in CF Patients
2.4. Chronic Inflammatory and Autoimmune Disorders
2.4.1. Limiting the Use of Gelsolin as a Disease Biomarker in Chronic Inflammatory Conditions
2.4.2. Uncertain Significance of Gelsolin as a Predictor in Autoimmune Diseases
2.5. Neurological and Neurodegenerative Diseases
2.5.1. Neuroprotective Role of Gelsolin in Ischemia-Induced Brain Injuries
2.5.2. The Protective Role of Gelsolin in Alzheimer’s Disease and Its Potential as an Indicator of Rapidity of Cognitive Decline
2.5.3. Pgsn-Mediated Limitation of Neuroinflammation as a Therapeutic Approach in Neurological Conditions
2.5.4. Proteolytic Cleavage of Gelsolin by Matrix Metalloproteinases in Subarachnoid Hemorrhage Patients
2.6. Cancer
2.6.1. Contradictory Effects and Expression of Gelsolin in Cancers
2.6.2. The Usefulness of pGSN in Distinguishing Cancers from Chronic Diseases and as a Predictor of Therapy Outcome
2.6.3. The Utility of Other pGSN-Containing Body Fluids in an Early Detection of Cancers
2.7. Chronic Kidney Diseases
2.7.1. The Impact of Decreased Levels of Gelsolin in Development of Kidney Diseases
2.7.2. pGSN as a Pathological Factor in IgA Nephropathy
2.8. Other Diseases
2.8.1. The Beneficial Role of Intracellular Gelsolin in Maintenance of Physiology of Pancreatic β-Cells: Anti-Diabetic Effect of Recombinant Gelsolin in an In Vivo Model of Diabetes
2.8.2. Changes of Gelsolin Concentration during Pregnancy
2.8.3. Impact of Reduced Levels of pGSN in Pre-Eclampsia Progression
2.8.4. Elevated Levels of Gelsolin in Rhabdomyolysis and Gelsolin-Related Familial Amyloidosis of Finnish Type
2.8.5. Gelsolin as a Universal Inflammatory Biomarker and Therapeutic Tool: Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, H.L.; Kwiatkowski, D.J.; Mole, J.E.; Cole, F.S. Structure and biosynthesis of cytoplasmic and secreted variants of gelsolin. J. Biol. Chem. 1984, 259, 5271–5276. [Google Scholar] [PubMed]
- Bucki, R.; Levental, I.; Kulakowska, A.; Janmey, P.A. Plasma gelsolin: Function, prognostic value, and potential therapeutic use. Curr. Protein Pept. Sci. 2008, 9, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Silacci, P.; Mazzolai, L.; Gauci, C.; Stergiopulos, N.; Yin, H.L.; Hayoz, D. Gelsolin superfamily proteins: Key regulators of cellular functions. Cell Mol. Life Sci. 2004, 61, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.G. Functional consequences of disulfide bond formation in gelsolin. FEBS Lett. 1997, 401, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Wen, D.; Corina, K.; Chow, E.P.; Miller, S.; Janmey, P.A.; Pepinsky, R.B. The plasma and cytoplasmic forms of human gelsolin differ in disulfide structure. Biochemistry 1996, 35, 9700–9709. [Google Scholar] [CrossRef] [PubMed]
- Vouyiouklis, D.A.; Brophy, P.J. A novel gelsolin isoform expressed by oligodendrocytes in the central nervous system. J. Neurochem. 1997, 69, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Cowin, A.J.; Lei, N.; Franken, L.; Ruzehaji, N.; Offenhäuser, C.; Kopecki, Z.; Murray, R.Z. Lysosomal secretion of flightless i upon injury has the potential to alter inflammation. Commun. Integr. Biol. 2012, 5, 546–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.B.; Janmey, P.A.; Herbert, T.J.; Lind, S.E. Quantitative measurement of plasma gelsolin and its incorporation into fibrin clots. J. Lab. Clin. Med. 1987, 110, 189–195. [Google Scholar] [PubMed]
- Kwiatkowski, D.J.; Mehl, R.; Izumo, S.; Nadal-Ginard, B.; Yin, H.L. Muscle is the major source of plasma gelsolin. J. Biol. Chem. 1988, 263, 8239–8243. [Google Scholar] [PubMed]
- Candiano, G.; Bruschi, M.; Pedemonte, N.; Caci, E.; Liberatori, S.; Bini, L.; Pellegrini, C.; Viganò, M.; O’Connor, B.J.; Lee, T.H.; et al. Gelsolin secretion in interleukin-4-treated bronchial epithelia and in asthmatic airways. Am. J. Respir. Crit. Care Med. 2005, 172, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.R.; Moore, E.E.; Damle, S.S.; Eckels, P.; Johnson, J.L.; Roach, J.P.; Redzic, J.S.; Hansen, K.C.; Banerjee, A. Gelsolin is depleted in post-shock mesenteric lymph. J. Surg. Res. 2007, 143, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F.; Baxter, C.R.; Zhu, M.; Yin, H.L. Detection of the actin scavenger system in burn wound fluid. Wound Repair. Regen. 1993, 1, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Desiderio, D.M. Proteomics analysis of phosphotyrosyl-proteins in human lumbar cerebrospinal fluid. J. Proteome Res. 2003, 2, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, T.; Pottiez, G.; Fox, H.S.; Ciborowski, P. Plasma gelsolin accumulates in macrophage nodules in brains of simian immunodeficiency virus infected rhesus macaques. J. Neurovirol. 2012, 18, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezen, D.; Bongiovanni, A.M.; Gelber, S.; Perni, U.; Hutson, J.M.; Skupski, D.; Witkin, S.S. Gelsolin down-regulates lipopolysaccharide-induced intraamniotic tumor necrosis factor-alpha production in the midtrimester of pregnancy. Am. J. Obstet. Gynecol. 2009, 200, 191.e1–191.e4. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, D.J. Functions of gelsolin: Motility, signaling, apoptosis, cancer. Curr. Opin. Cell Biol. 1999, 11, 103–108. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, B.; Chen, Q.; Wu, S.; Lv, C.; Xie, G.; Jin, Y.; Fang, X. Time course of plasma gelsolin concentrations during severe sepsis in critically ill surgical patients. Crit. Care 2008, 12, R106. [Google Scholar] [CrossRef] [PubMed]
- Mounzer, K.C.; Moncure, M.; Smith, Y.R.; Dinubile, M.J. Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am. J. Respir. Crit. Care Med. 1999, 160, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Janmey, P.A. Extracellular aggregation of polyelectrolytes escaped from the cell interior: Mechanisms and physiological consequences. Curr. Opin. Colloid Interface Sci. 2016, 26, 84–89. [Google Scholar] [CrossRef]
- Lee, W.M.; Galbraith, R.M. The extracellular actin-scavenger system and actin toxicity. N. Engl. J. Med. 1992, 326, 1335–1341. [Google Scholar] [PubMed]
- Haddad, J.G.; Harper, K.D.; Guoth, M.; Pietra, G.G.; Sanger, J.W. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc. Natl. Acad. Sci. USA 1990, 87, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Basaraba, R.J.; Byerly, A.N.; Stewart, G.C.; Mosier, D.A.; Fenwick, B.W.; Chengappa, M.M.; Laegreid, W.W. Actin enhances the haemolytic activity of escherichia coli. Microbiology 1998, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, C.A.; Lind, S.E. Coordinated inhibition of actin-induced platelet aggregation by plasma gelsolin and vitamin d-binding protein. Blood 1993, 82, 3648–3657. [Google Scholar] [PubMed]
- Scarborough, V.D.; Bradford, H.R.; Ganguly, P. Aggregation of platelets by muscle actin. A multivalent interaction model of platelet aggregation by adp. Biochem. Biophys. Res. Commun. 1981, 100, 1314–1319. [Google Scholar] [CrossRef]
- Bucki, R.; Byfield, F.J.; Kulakowska, A.; McCormick, M.E.; Drozdowski, W.; Namiot, Z.; Hartung, T.; Janmey, P.A. Extracellular gelsolin binds lipoteichoic acid and modulates cellular response to proinflammatory bacterial wall components. J. Immunol. 2008, 181, 4936–4944. [Google Scholar] [CrossRef] [PubMed]
- Osborn, T.M.; Dahlgren, C.; Hartwig, J.H.; Stossel, T.P. Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin. Am. J. Physiol. Cell. Physiol. 2007, 292, C1323–C1330. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Kulakowska, A.; Byfield, F.J.; Zendzian-Piotrowska, M.; Baranowski, M.; Marzec, M.; Winer, J.P.; Ciccarelli, N.J.; Górski, J.; Drozdowski, W.; et al. Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am. J. Physiol. Cell. Physiol. 2010, 299, C1516–C1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, S.E.; Janmey, P.A. Human plasma gelsolin binds to fibronectin. J. Biol. Chem. 1984, 259, 13262–13266. [Google Scholar] [PubMed]
- Ruzehaji, N.; Mills, S.J.; Melville, E.; Arkell, R.; Fitridge, R.; Cowin, A.J. The influence of flightless i on toll-like-receptor-mediated inflammation in a murine model of diabetic wound healing. Biomed. Res. Int. 2013, 2013, 389792. [Google Scholar] [CrossRef] [PubMed]
- Pellieux, C.; Desgeorges, A.; Pigeon, C.H.; Chambaz, C.; Yin, H.; Hayoz, D.; Silacci, P. Cap g, a gelsolin family protein modulating protective effects of unidirectional shear stress. J. Biol. Chem. 2003, 278, 29136–29144. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.; Jiang, H.; Wang, Y.; Kuiper, J.W.; Glogauer, M. The actin binding protein adseverin regulates osteoclastogenesis. PLoS ONE 2014, 9, e109078. [Google Scholar] [CrossRef] [PubMed]
- Witke, W.; Sharpe, A.H.; Hartwig, J.H.; Azuma, T.; Stossel, T.P.; Kwiatkowski, D.J. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell 1995, 81, 41–51. [Google Scholar] [CrossRef]
- Furukawa, K.; Fu, W.; Li, Y.; Witke, W.; Kwiatkowski, D.J.; Mattson, M.P. The actin-severing protein gelsolin modulates calcium channel and nmda receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J. Neurosci. 1997, 17, 8178–8186. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Parasar, D.; Sagar, A.; Choudhary, V.; Chopra, B.S.; Garg, R.; Khatri, N. Analgesic and anti-inflammatory properties of gelsolin in acetic acid induced writhing, tail immersion and carrageenan induced paw edema in mice. PLoS ONE 2015, 10, e0135558. [Google Scholar] [CrossRef] [PubMed]
- Li-ChunHsieh, K.; Schob, S.; Zeller, M.W.; Pulli, B.; Ali, M.; Wang, C.; Chiou, T.T.; Tsang, Y.M.; Lee, P.S.; Stossel, T.P.; et al. Gelsolin decreases actin toxicity and inflammation in murine multiple sclerosis. J. Neuroimmunol 2015, 287, 36–42. [Google Scholar] [PubMed] [Green Version]
- Khatri, N.; Sagar, A.; Peddada, N.; Choudhary, V.; Chopra, B.S.; Garg, V.; Garg, R. Plasma gelsolin levels decrease in diabetic state and increase upon treatment with f-actin depolymerizing versions of gelsolin. J. Diabetes. Res. 2014, 2014, 152075. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.; Kizer, N.; Silva, M.; Alvarez, U.; Kwiatkowski, D.; Hruska, K.A. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J. Cell. Biol. 2000, 148, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Rothenbach, P.A.; Dahl, B.; Schwartz, J.J.; O’Keefe, G.E.; Yamamoto, M.; Lee, W.M.; Horton, J.W.; Yin, H.L.; Turnage, R.H. Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J. Appl. Physiol. (1985) 2004, 96, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christofidou-Solomidou, M.; Scherpereel, A.; Solomides, C.C.; Muzykantov, V.R.; Machtay, M.; Albelda, S.M.; DiNubile, M.J. Changes in plasma gelsolin concentration during acute oxidant lung injury in mice. Lung 2002, 180, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Liu, W.G.; Dong, X.Q.; Yang, S.B.; Fan, J. Change in plasma gelsolin level after traumatic brain injury. J. Trauma. Acute. Care Surg. 2012, 72, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Dahl, B.; Schiødt, F.V.; Ott, P.; Gvozdenovic, R.; Yin, H.L.; Lee, W.M. Plasma gelsolin is reduced in trauma patients. Shock 1999, 12, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, F.; Cheng, Y.; Han, L.; Wang, J.; Sun, D.; Liu, Y.L.; Zhou, P.K.; Min, R. Gelsolin: Role of a functional protein in mitigating radiation injury. Cell. Biochem. Biophys. 2015, 71, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Rithidech, K.N.; Reungpatthanaphong, P.; Tungjai, M.; Jangiam, W.; Honikel, L.; Whorton, E.B. Persistent depletion of plasma gelsolin (pgsn) after exposure of mice to heavy silicon ions. Life Sci. Space. Res. 2018, 17, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Suhler, E.; Lin, W.; Yin, H.L.; Lee, W.M. Decreased plasma gelsolin concentrations in acute liver failure, myocardial infarction, septic shock, and myonecrosis. Crit. Care Med. 1997, 25, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Erukhimov, J.A.; Tang, Z.L.; Johnson, B.A.; Donahoe, M.P.; Razzack, J.A.; Gibson, K.F.; Lee, W.M.; Wasserloos, K.J.; Watkins, S.A.; Pitt, B.R. Actin-containing sera from patients with adult respiratory distress syndrome are toxic to sheep pulmonary endothelial cells. Am. J. Respir. Crit. Care Med. 2000, 162, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Drager, L.R.; Stossel, T.P.; Moore, F.D.; Rogers, S.O. Relationship of plasma gelsolin levels to outcomes in critically ill surgical patients. Ann. Surg. 2006, 243, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Xianhui, L.; Pinglian, L.; Xiaojuan, W.; Wei, C.; Yong, Y.; Feng, R.; Peng, S.; Gang, X. The association between plasma gelsolin level and prognosis of burn patients. Burns 2014, 40, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Yao, Y.M.; Li, J.F.; Dong, N.; Liu, C.; Yu, Y.; He, L.X.; Sheng, Z.Y. Reduction of plasma gelsolin levels correlates with development of multiple organ dysfunction syndrome and fatal outcome in burn patients. PLoS ONE 2011, 6, e25748. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Meng, K.; Su, W.; Fu, Y. The effect of continuous sedation therapy on immunomodulation, plasma levels of antioxidants, and indicators of tissue repair in post-burn sepsis patients. Cell. Biochem. Biophys. 2015, 73, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, B.Y.; Qiu, L.L.; Ling, Y.R.; Bai, Z.Q. Decreased plasma gelsolin is associated with 1-year outcome in patients with traumatic brain injury. J. Crit. Care 2012, 27, e521–e526. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Chen, C.; Zhao, D.; Liu, X.; Cheng, B.; Wu, S.; Lin, R.; Tan, L.; Fang, X.; Shu, Q. The role of plasma gelsolin in cardiopulmonary bypass induced acute lung injury in infants and young children: A. pilot study. BMC. Anesthesiol 2014, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- DiNubile, M.J.; Stossel, T.P.; Ljunghusen, O.C.; Ferrara, J.L.; Antin, J.H. Prognostic implications of declining plasma gelsolin levels after allogeneic stem cell transplantation. Blood 2002, 100, 4367–4371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Kambe, H.; Kimura, Y.; Nakamura, H.; Hayashi, E.; Kishimoto, T.; Kishimoto, S.; Yamamoto, H. Depression of plasma gelsolin level during acute liver injury. Gastroenterology 1992, 102, 1686–1692. [Google Scholar] [CrossRef]
- Halis, H.; Gunes, T.; Korkut, S.; Saraymen, B.; Şen, A.; Bastug, O.; Öztürk, A.; Kurtoğlu, S. In the diagnosis of neonatal sepsis importance of gelsolin and relationship with mortality and morbidity. Med. Hypotheses 2016, 94, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Patel, S.R.; Christiani, D.C.; Bajwa, E.; Stossel, T.P.; Waxman, A.B. Plasma gelsolin depletion and circulating actin in sepsis: A. pilot study. PLoS ONE 2008, 3, e3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.B.; Janmey, P.A.; Sherwood, J.A.; Howard, R.J.; Lind, S.E. Decreased plasma gelsolin levels in patients with plasmodium falciparum malaria: A consequence of hemolysis? Blood 1988, 72, 214–218. [Google Scholar] [PubMed]
- Kassa, F.A.; Shio, M.T.; Bellemare, M.J.; Faye, B.; Ndao, M.; Olivier, M. New inflammation-related biomarkers during malaria infection. PLoS ONE 2011, 6, e26495. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, C.; Rinalducci, S.; Mohamadkhani, A.; D’Amici, G.M.; Zolla, L. Plasma gelsolin protein: A candidate biomarker for hepatitis b-associated liver cirrhosis identified by proteomic approach. Blood Transfus. 2010, 8, s105–s112. [Google Scholar] [PubMed]
- Rozek, W.; Ricardo-Dukelow, M.; Holloway, S.; Gendelman, H.E.; Wojna, V.; Melendez, L.M.; Ciborowski, P. Cerebrospinal fluid proteomic profiling of hiv-1-infected patients with cognitive impairment. J. Proteome Res. 2007, 6, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Wiederin, J.; Rozek, W.; Duan, F.; Ciborowski, P. Biomarkers of hiv-1 associated dementia: Proteomic investigation of sera. Proteome. Sci. 2009, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Osborn, T.M.; Verdrengh, M.; Stossel, T.P.; Tarkowski, A.; Bokarewa, M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R117. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Johnsson, H.; McInnes, I.B.; Husi, H.; Klein, J.; Dakna, M.; Mullen, W.; Mischak, H.; Porter, D. Identification of urinary peptide biomarkers associated with rheumatoid arthritis. PLoS ONE 2014, 9, e104625. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Yoo, S.A.; Hwang, D.; Cho, C.S.; Kim, W.U. Identification of novel urinary biomarkers for assessing disease activity and prognosis of rheumatoid arthritis. Exp. Mol. Med. 2016, 48, e211. [Google Scholar] [CrossRef] [PubMed]
- Eke Gungor, H.; Sahiner, U.M.; Karakukcu, C.; Sahiner, N.; Altuner Torun, Y. The plasma gelsolin levels in atopic dermatitis: Effect of atopy and disease severity. Allergol. Immunopathol. (Madr) 2016, 44, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hosseini, A.; Kauwe, J.S.; Gross, J.; Cairns, N.J.; Goate, A.M.; Fagan, A.M.; Townsend, R.R.; Holtzman, D.M. Identification and validation of novel csf biomarkers for early stages of alzheimer’s disease. Proteomics. Clin. Appl. 2007, 1, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Kułakowska, A.; Zajkowska, J.M.; Ciccarelli, N.J.; Mroczko, B.; Drozdowski, W.; Bucki, R. Depletion of plasma gelsolin in patients with tick-borne encephalitis and lyme neuroborreliosis. Neurodegener Dis. 2011, 8, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Kułakowska, A.; Ciccarelli, N.J.; Wen, Q.; Mroczko, B.; Drozdowski, W.; Szmitkowski, M.; Janmey, P.A.; Bucki, R. Hypogelsolinemia, a disorder of the extracellular actin scavenger system, in patients with multiple sclerosis. BMC. Neurol. 2010, 10, 107. [Google Scholar]
- Zhao, D.Q.; Wang, K.; Zhang, H.D.; Li, Y.J. Significant reduction of plasma gelsolin levels in patients with intracerebral hemorrhage. Clin. Chim. Acta. 2013, 415, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Lo, E.H.; Ning, M. Plasma-type gelsolin in subarachnoid hemorrhage: Novel biomarker today, therapeutic target tomorrow? Crit. Care 2014, 18, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, S.H.; Lee, P.S.; Konigsberg, R.G.; Gallacci, D.; Chiou, T.; Arai, K.; Simmons, S.; Bauer, D.; Feske, S.K.; Lo, E.H.; et al. Plasma-type gelsolin is decreased in human blood and cerebrospinal fluid after subarachnoid hemorrhage. Stroke 2011, 42, 3624–3627. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Wu, C.C.; Peng, P.H.; Liang, Y.; Hsiao, Y.C.; Chien, K.Y.; Chen, J.T.; Lin, S.J.; Tang, R.P.; Hsieh, L.L.; et al. Identification of secretory gelsolin as a plasma biomarker associated with distant organ metastasis of colorectal cancer. J. Mol. Med. (Berl) 2012, 90, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Chen, R.; Brand, R.E.; Hawley, S.; Tamura, Y.; Gafken, P.R.; Milless, B.P.; Goodlett, D.R.; Rush, J.; Brentnall, T.A. Multiplex targeted proteomic assay for biomarker detection in plasma: A pancreatic cancer biomarker case study. J. Proteome Res. 2012, 11, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Scumaci, D.; Tammè, L.; Fiumara, C.V.; Pappaianni, G.; Concolino, A.; Leone, E.; Faniello, M.C.; Quaresima, B.; Ricevuto, E.; Costanzo, F.S.; et al. Plasma proteomic profiling in hereditary breast cancer reveals a brca1-specific signature: Diagnostic and functional implications. PLoS ONE 2015, 10, e0129762. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Matsumoto, T.; Nagashio, R.; Kageyama, T.; Utsuki, S.; Oka, H.; Okayasu, I.; Sato, Y. Proteomics of tumor-specific proteins in cerebrospinal fluid of patients with astrocytoma: Usefulness of gelsolin protein. Pathol. Int. 2009, 59, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Sampath, K.; Karumanchi, S.A.; Tamez, H.; Bhan, I.; Isakova, T.; Gutierrez, O.M.; Wolf, M.; Chang, Y.; Stossel, T.P.; et al. Plasma gelsolin and circulating actin correlate with hemodialysis mortality. J. Am. Soc. Nephrol. 2009, 20, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.T.; Liao, S.C.; Kao, Y.Y.; Lee, W.C.; Lee, Y.T.; Ng, H.Y.; Lee, P.S.; Lee, C.T. Gelsolin and progression of aortic arch calcification in chronic hemodialysis patients. Int. J. Med. Sci. 2016, 13, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fillmore, T.L.; Schepmoes, A.A.; Clauss, T.R.; Gritsenko, M.A.; Mueller, P.W.; Rewers, M.; Atkinson, M.A.; Smith, R.D.; Metz, T.O. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. J. Exp. Med. 2013, 210, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Min, H.; Kim, S.J.; Oh, S.; Kim, K.; Yu, H.G.; Park, T.; Kim, Y. Development of diagnostic biomarkers for detecting diabetic retinopathy at early stages using quantitative proteomics. J. Diabetes Res. 2016, 2016, 6571976. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, N.A.; Rajakumar, A.; Mokhashi, N.; Burke, S.D.; Rana, S.; Salahuddin, S.; Dang, Q.; Thadhani, R.; Krishnan, R.; Stossel, T.P.; et al. Gelsolin is an endogenous inhibitor of syncytiotrophoblast extracellular vesicle shedding in pregnancy. Pregnancy Hypertens 2016, 6, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tannetta, D.S.; Redman, C.W.; Sargent, I.L. Investigation of the actin scavenging system in pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod Biol. 2014, 172, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Löfberg, M.; Paunio, T.; Tähtelä, R.; Kiuru, S.; Somer, H. Serum gelsolin and rhabdomyolysis. J. Neurol. Sci. 1998, 157, 187–190. [Google Scholar] [CrossRef]
- Weeds, A.G.; Gooch, J.; McLaughlin, P.; Maury, C.P. Variant plasma gelsolin responsible for familial amyloidosis (finnish type) has defective actin severing activity. FEBS. Lett 1993, 335, 119–123. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Scherpereel, A.; Solomides, C.C.; Christie, J.D.; Stossel, T.P.; Goelz, S.; DiNubile, M.J. Recombinant plasma gelsolin diminishes the acute inflammatory response to hyperoxia in mice. J. Investig. Med. 2002, 50, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Chen, Q.; Kang, J.R.; Liu, C.; Dong, N.; Zhu, X.M.; Sheng, Z.Y.; Yao, Y.M. Treatment with gelsolin reduces brain inflammation and apoptotic signaling in mice following thermal injury. J. Neuroinflamm. 2011, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.S.; Waxman, A.B.; Cotich, K.L.; Chung, S.W.; Perrella, M.A.; Stossel, T.P. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit. Care Med. 2007, 35, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.S.; Bucki, R.; Byfield, F.J.; Ciccarelli, N.J.; Rosenberg, B.; DiNubile, M.J.; Janmey, P.A.; Margulies, S.S. Therapeutic potential of plasma gelsolin administration in a rat model of sepsis. Cytokine 2011, 54, 235–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Chiou, T.T.; Stossel, T.P.; Kobzik, L. Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage nos3 function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L11–L16. [Google Scholar] [CrossRef] [PubMed]
- Hirko, A.C.; Meyer, E.M.; King, M.A.; Hughes, J.A. Peripheral transgene expression of plasma gelsolin reduces amyloid in transgenic mouse models of alzheimer’s disease. Mol. Ther. 2007, 15, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Saito, M.; LaFrancois, J.; Gaynor, K.; Olm, V.; Wang, L.; Casey, E.; Lu, Y.; Shiratori, C.; Lemere, C.; et al. Novel therapeutic approach for the treatment of alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J. Neurosci. 2003, 23, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Janmey, P.A.; Lind, S.E. Circulating actin-gelsolin complexes following oleic acid-induced lung injury. Am. J. Pathol. 1988, 130, 261–267. [Google Scholar] [PubMed]
- Lind, S.E.; Smith, D.B.; Janmey, P.A.; Stossel, T.P. Depression of gelsolin levels and detection of gelsolin-actin complexes in plasma of patients with acute lung injury. Am. Rev. Respir. Dis. 1988, 138, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.M.; Kazi, A.A.; Wadgaonkar, R.; Pearse, D.B.; Kwiatkowski, D.; Garcia, J.G. Pulmonary vascular permeability and ischemic injury in gelsolin-deficient mice. Am. J. Respir. Cell. Mol. Biol. 2003, 28, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Degoricija, V.; Sharma, M.; Legac, A.; Gradiser, M.; Sefer, S.; Vucicević, Z. Survival analysis of 314 episodes of sepsis in medical intensive care unit in university hospital: Impact of intensive care unit performance and antimicrobial therapy. Croat. Med. J. 2006, 47, 385–397. [Google Scholar] [PubMed]
- Gullo, A.; Bianco, N.; Berlot, G. Management of severe sepsis and septic shock: Challenges and recommendations. Crit. Care Clin. 2006, 22, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Vincent, J.L. Sepsis biomarkers: A. review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Schmit, X.; Vincent, J.L. The time course of blood c-reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection 2008, 36, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Durnaś, B.; Piktel, E.; Wątek, M.; Wollny, T.; Góźdź, S.; Smok-Kalwat, J.; Niemirowicz, K.; Savage, P.B.; Bucki, R. Anaerobic bacteria growth in the presence of cathelicidin ll-37 and selected ceragenins delivered as magnetic nanoparticles cargo. BMC Microbiol. 2017, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Durnaś, B.; Wątek, M.; Wollny, T.; Niemirowicz, K.; Marzec, M.; Bucki, R.; Góźdź, S. Utility of blood procalcitonin concentration in the management of cancer patients with infections. Onco. Targets. Ther. 2016, 9, 469–475. [Google Scholar] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Olshaker, J.S. The c-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Craig, J.C.; McLean, A.S. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet. Infect. Dis. 2007, 7, 210–217. [Google Scholar] [CrossRef]
- Horváth-Szalai, Z.; Kustán, P.; Mühl, D.; Ludány, A.; Bugyi, B.; Kőszegi, T. Antagonistic sepsis markers: Serum gelsolin and actin/gelsolin ratio. Clin. Biochem. 2017, 50, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rhoads, S.L.; DiNubile, M.J. Temporal association between serum gelsolin levels and clinical events in a patient with severe falciparum malaria. Clin. Infect. Dis. 1997, 24, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Horváth-Szalai, Z.; Kustán, P.; Szirmay, B.; Lakatos, Á.; Christensen, P.H.; Huber, T.; Bugyi, B.; Mühl, D.; Ludány, A.; Miseta, A.; et al. Validation of an automated immune turbidimetric assay for serum gelsolin and its possible clinical utility in sepsis. J. Clin. Lab. Anal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Horváth-Szalai, Z.; Kustán, P.; Szirmay, B.; Lakatos, Á.; Christensen, P.H.; Huber, T.; Bugyi, B.; Mühl, D.; Ludány, A.; Miseta, A.; et al. Predictive value of serum gelsolin and gc globulin in sepsis—A pilot study. Clin. Chem. Lab. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Pastore, J.J.; Randhawa, P.; Vegners, R.; Weiner, D.J.; Janmey, P.A. Antibacterial activities of rhodamine b-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin ll37, magainin ii, and melittin. Antimicrob. Agents Chemother. 2004, 48, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Georges, P.C.; Espinassous, Q.; Funaki, M.; Pastore, J.J.; Chaby, R.; Janmey, P.A. Inactivation of endotoxin by human plasma gelsolin. Biochemistry 2005, 44, 9590–9597. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Janmey, P.A. Interaction of the gelsolin-derived antibacterial pbp 10 peptide with lipid bilayers and cell membranes. Antimicrob. Agents Chemother. 2006, 50, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. Lps induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, X.; Liu, C.; Chen, M.; Wang, J.; Wang, M.; Gao, F.; Han, J.; Zhang, C.; Sun, D.; et al. Gelsolin inhibits the inflammatory process induced by lps. Cell. Physiol. Biochem. 2017, 41, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Poltorak, A. Sepsis and evolution of the innate immune response. Crit. Care Med. 2001, 29, S2–S6. [Google Scholar] [CrossRef]
- Serrander, L.; Skarman, P.; Rasmussen, B.; Witke, W.; Lew, D.P.; Krause, K.H.; Stendahl, O.; Nüsse, O. Selective inhibition of igg-mediated phagocytosis in gelsolin-deficient murine neutrophils. J. Immunol. 2000, 165, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Glogauer, M.; Kapus, A.; Kwiatkowski, D.J.; McCulloch, C.A. Gelsolin mediates collagen phagocytosis through a rac-dependent step. Mol. Biol. Cell. 2004, 15, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Ordija, C.M.; Chiou, T.T.; Yang, Z.; Deloid, G.M.; de Oliveira Valdo, M.; Wang, Z.; Bedugnis, A.; Noah, T.L.; Jones, S.; Koziel, H.; et al. Free actin impairs macrophage bacterial defenses via scavenger receptor marco interaction with reversal by plasma gelsolin. Am. J. Physiol Lung Cell. Mol. Physiol 2017, 312, L1018–L1028. [Google Scholar] [CrossRef] [PubMed]
- Argun, M.; Baykan, A.; Narin, F.; Özyurt, A.; Pamukçu, Ö.; Elmalı, F.; Üzüm, K.; Narin, N. Plasma gelsolin as a biomarker of acute rheumatic carditis. Cardiol. Young 2015, 25, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.J.; Garg, N.J. Proteome expression and carbonylation changes during trypanosoma cruzi infection and chagas disease in rats. Mol. Cell. Proteom. 2012, 11, M111.010918. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Levinson, S.; Stossel, T.; DiNubile, M.; Kobzik, L. Delayed therapy with plasma gelsolin improves survival in murine pneumococcal pneumonia. Open Forum Infect. Dis. 2017, 474–475. [Google Scholar] [CrossRef]
- García-Expósito, L.; Ziglio, S.; Barroso-González, J.; de Armas-Rillo, L.; Valera, M.S.; Zipeto, D.; Machado, J.D.; Valenzuela-Fernández, A. Gelsolin activity controls efficient early hiv-1 infection. Retrovirology 2013, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; McMillan, J.R. Gelsolin segment 5 inhibits hiv-induced t-cell apoptosis via vpr-binding to vdac. FEBS Lett. 2007, 581, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Liang, S.; Xiong, H. Plasma gelsolin protects hiv-1 gp120-induced neuronal injury via voltage-gated k+ channel kv2.1. Mol. Cell. Neurosci. 2013, 57, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Boukli, N.M.; Shetty, V.; Cubano, L.; Ricaurte, M.; Coelho-Dos-Reis, J.; Nickens, Z.; Shah, P.; Talal, A.H.; Philip, R.; Jain, P. Unique and differential protein signatures within the mononuclear cells of hiv-1 and hcv mono-infected and co-infected patients. Clin. Proteom. 2012, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Whitby, P.W.; Dick, H.L.; Campbell, P.W.; Tullis, D.E.; Matlow, A.; Stull, T.L. Comparison of culture and pcr for detection of burkholderia cepacia in sputum samples of patients with cystic fibrosis. J. Clin. Microbiol. 1998, 36, 1642–1645. [Google Scholar] [PubMed]
- DENTON, R. Bronchial obstruction in cystic fibrosis: Rheological factors. Pediatrics 1960, 25, 611–620. [Google Scholar] [PubMed]
- Bucki, R.; Byfield, F.J.; Janmey, P.A. Release of the antimicrobial peptide ll-37 from dna/f-actin bundles in cystic fibrosis sputum. Eur. Respir. J. 2007, 29, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Cruz, K.; Pogoda, K.; Eggert, A.; Chin, L.; Ferrin, M.; Imbesi, G.; Hadjiliadis, D.; Janmey, P.A. Enhancement of pulmozyme activity in purulent sputum by combination with poly-aspartic acid or gelsolin. J. Cyst. Fibros. 2015, 14, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.S.; Tomlin, K.L.; Worthen, G.S.; Poch, K.R.; Lieber, J.G.; Saavedra, M.T.; Fessler, M.B.; Malcolm, K.C.; Vasil, M.L.; Nick, J.A. Enhanced pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect. Immun. 2005, 73, 3693–3701. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Schradin, C. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public Health 2016, 2016, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Park, Y.J.; You, S.; Yoo, S.A.; Choi, S.; Kim, D.H.; Cho, C.S.; Yi, E.C.; Hwang, D.; Kim, W.U. Urinary proteome profile predictive of disease activity in rheumatoid arthritis. J. Proteome Res. 2014, 13, 5206–5217. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, H.; Li, W.H.; Meng, H.X.; Fan, Y.Z.; Li, W.J.; Ji, Y.T.; Zhao, H.; Zhang, L.; Jin, X.M.; et al. The value of decreased plasma gelsolin levels in patients with systemic lupus erythematosus and rheumatoid arthritis in diagnosis and disease activity evaluation. Lupus 2013, 22, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Genre, F.; López-Mejías, R.; Miranda-Filloy, J.A.; Ubilla, B.; Carnero-López, B.; Gómez-Acebo, I.; Blanco, R.; Ochoa, R.; Rueda-Gotor, J.; González-Juanatey, C.; et al. Gelsolin levels are decreased in ankylosing spondylitis patients undergoing anti-tnf-alpha therapy. Clin. Exp. Rheumatol 2014, 32, 218–224. [Google Scholar] [PubMed]
- Janciauskiene, S.; Olejnicka, B.; Koczulla, R.; Cardell, L.O.; Welte, T.; Westin, U. Allergen-specific immunotherapy increases plasma gelsolin levels. Am. J. Rhinol. Allergy. 2014, 28, e136–e140. [Google Scholar] [CrossRef] [PubMed]
- Kandur, Y.; Çelik, A.; Gözübenli, F.; Çetinkaya, A.; Olgar, Ş. Plasma gelsolin as a potential biomarker for henoch-schoenlein purpura. Scand. J. Rheumatol. 2017, 46, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.; Fink, K.; Zhu, J.; Stagliano, N.E.; Bondada, V.; Geddes, J.W.; Azuma, T.; Mattson, M.P.; Kwiatkowski, D.J.; Moskowitz, M.A. Neuroprotective effects of gelsolin during murine stroke. J. Clin. Investig. 1999, 103, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carro, E. Gelsolin as therapeutic target in alzheimer’s disease. Expert Opin. Ther. Targets 2010, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; He, L.N.; Xiao, F.; Shen, J.; Zhan, R.Y. Plasma gelsolin levels and outcomes after aneurysmal subarachnoid hemorrhage. Crit. Care 2013, 17, R149. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, F.; Gertz, K.; Kronenberg, G.; Harms, C.; Fink, K.B.; Meisel, A.; Endres, M. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp. Neurol. 2008, 210, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Ji, L.; Chauhan, A. Anti-amyloidogenic, anti-oxidant and anti-apoptotic role of gelsolin in alzheimer’s disease. Biogerontology 2008, 9, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kothakota, S.; Azuma, T.; Reinhard, C.; Klippel, A.; Tang, J.; Chu, K.; McGarry, T.J.; Kirschner, M.W.; Koths, K.; Kwiatkowski, D.J.; et al. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science 1997, 278, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Ray, I.; Chauhan, A.; Wisniewski, H.M. Binding of gelsolin, a secretory protein, to amyloid beta-protein. Biochem. Biophys. Res. Commun. 1999, 258, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Koya, R.C.; Nakagawa, K.; Tanaka, H.; Fujita, H.; Takimoto, M.; Kuzumaki, N. Inhibition of alzheimer’s amyloid-beta peptide-induced reduction of mitochondrial membrane potential and neurotoxicity by gelsolin. Neurobiol. Aging 2005, 26, 849–855. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, B.; Sheehan, D. Effect of oxidative stress on protein thiols in the blue mussel mytilus edulis: Proteomic identification of target proteins. Proteomics 2007, 7, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Güntert, A.; Campbell, J.; Saleem, M.; O’Brien, D.P.; Thompson, A.J.; Byers, H.L.; Ward, M.A.; Lovestone, S. Plasma gelsolin is decreased and correlates with rate of decline in alzheimer’s disease. J. Alzheimers Dis. 2010, 21, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chauhan, A.; Wegiel, J.; Essa, M.M.; Chauhan, V. Gelsolin is proteolytically cleaved in the brains of individuals with alzheimer’s disease. J. Alzheimers Dis. 2009, 18, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, A.; Mishra, M.; Feng, L.; Sze, S.K.; Akatsu, H.; Heese, K. Brain site-specific proteome changes in aging-related dementia. Exp. Mol. Med. 2013, 45, e39. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.E.; Dammer, E.B.; Duong, D.M.; Hanfelt, J.J.; Levey, A.I.; Seyfried, N.T.; Lah, J.J. Exploring the potential of the platelet membrane proteome as a source of peripheral biomarkers for alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Jia, J.; Qin, W. Plasma gelsolin and matrix metalloproteinase 3 as potential biomarkers for alzheimer disease. Neurosci. Lett. 2015, 595, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple sclerosis and neuroinflammation: The overview of current and prospective therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef] [PubMed]
- Forghani, R.; Wojtkiewicz, G.R.; Zhang, Y.; Seeburg, D.; Bautz, B.R.; Pulli, B.; Milewski, A.R.; Atkinson, W.L.; Iwamoto, Y.; Zhang, E.R.; et al. Demyelinating diseases: Myeloperoxidase as an imaging biomarker and therapeutic target. Radiology 2012, 263, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Hwang, I.K.; Kim, S.Y.; Lee, S.J.; Park, K.S.; Lee, S.T. Characterization of plasma gelsolin as a substrate for matrix metalloproteinases. Proteomics 2006, 6, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, Y.K.; Kwon, H.J.; Yang, H.K.; Choi, J.H.; Kim, D.Y. Downregulation of gelsolin and retinoic acid receptor beta expression in gastric cancer tissues through histone deacetylase 1. J. Gastroenterol. Hepatol. 2004, 19, 218–224. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, W.; Li, J.; Zheng, P.; Dong, P.; Chen, L.; Zhou, Y.; Xie, G.; Xu, D.; Liu, Y.; et al. Gelsolin suppresses gastric cancer metastasis through inhibition of pkr-p38 signaling. Oncotarget 2016, 7, 53459–53470. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Mazur, A.J.; Nowak, D.; Mannherz, H.G.; Malicka-Błaszkiewicz, M. Gelsolin in human colon adenocarcinoma cells with different metastatic potential. Acta. Biochim. Pol. 2009, 56, 739–743. [Google Scholar] [PubMed]
- Li, W.X.; Yang, M.X.; Hong, X.Q.; Dong, T.G.; Yi, T.; Lin, S.L.; Qin, X.Y.; Niu, W.X. Overexpression of gelsolin reduces the proliferation and invasion of colon carcinoma cells. Mol. Med. Rep. 2016, 14, 3059–3065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.G.; Zhou, L.; Wang, G.Q.; Liu, S.M.; Bai, X.F.; Liu, F.; Peppelenbosch, M.P.; Zhao, P. The ubiquitin-proteasome pathway mediates gelsolin protein downregulation in pancreatic cancer. Mol. Med. 2008, 14, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Ashcroft, F.J.; Patel, S.; Saraga, G.; Vimalachandran, D.; Prime, W.; Campbell, F.; Dodson, A.; Jenkins, R.E.; Lemoine, N.R.; et al. Pancreatic cancer cells overexpress gelsolin family-capping proteins, which contribute to their cell motility. Gut 2007, 56, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winston, J.S.; Asch, H.L.; Zhang, P.J.; Edge, S.B.; Hyland, A.; Asch, B.B. Downregulation of gelsolin correlates with the progression to breast carcinoma. Breast Cancer Res. Treat. 2001, 65, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.J.; Gao, C.F.; Wang, C.S.; Lv, J.J.; Zhao, G.; Sheng, X.H.; Wang, X.L.; Li, D.H.; Liu, Q.Y.; Yin, J. Discovery and verification of gelsolin as a potential biomarker of colorectal adenocarcinoma in the chinese population: Examining differential protein expression using an itraq labelling-based proteomics approach. Can. J. Gastroenterol. 2012, 26, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dosaka-Akita, H.; Hommura, F.; Fujita, H.; Kinoshita, I.; Nishi, M.; Morikawa, T.; Katoh, H.; Kawakami, Y.; Kuzumaki, N. Frequent loss of gelsolin expression in non-small cell lung cancers of heavy smokers. Cancer Res. 1998, 58, 322–327. [Google Scholar] [PubMed]
- Lee, H.K.; Driscoll, D.; Asch, H.; Asch, B.; Zhang, P.J. Downregulated gelsolin expression in hyperplastic and neoplastic lesions of the prostate. Prostate 1999, 40, 14–19. [Google Scholar] [CrossRef]
- Visapää, H.; Bui, M.; Huang, Y.; Seligson, D.; Tsai, H.; Pantuck, A.; Figlin, R.; Rao, J.Y.; Belldegrun, A.; Horvath, S.; et al. Correlation of ki-67 and gelsolin expression to clinical outcome in renal clear cell carcinoma. Urology 2003, 61, 845–850. [Google Scholar] [CrossRef]
- Noske, A.; Denkert, C.; Schober, H.; Sers, C.; Zhumabayeva, B.; Weichert, W.; Dietel, M.; Wiechen, K. Loss of gelsolin expression in human ovarian carcinomas. Eur. J. Cancer 2005, 41, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.; Tan, E.H.; Yan, B.; Tochhawng, L.; Jayapal, M.; Koh, S.; Tay, H.K.; Maciver, S.K.; Hooi, S.C.; Salto-Tellez, M.; et al. Gelsolin induces colorectal tumor cell invasion via modulation of the urokinase-type plasminogen activator cascade. PLoS ONE 2012, 7, e43594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Ramnath, N.; Moysich, K.B.; Asch, H.L.; Swede, H.; Alrawi, S.J.; Huberman, J.; Geradts, J.; Brooks, J.S.; Tan, D. Prognostic significance of mcm2, ki-67 and gelsolin in non-small cell lung cancer. BMC Cancer 2006, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Hao, J.; Han, W.; Ni, Y.; Huang, X.; Hu, Q. Gelsolin regulates proliferation, apoptosis, migration and invasion in human oral carcinoma cells. Oncol. Lett. 2015, 9, 2129–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Corte, V.; Bruyneel, E.; Boucherie, C.; Mareel, M.; Vandekerckhove, J.; Gettemans, J. Gelsolin-induced epithelial cell invasion is dependent on ras-rac signaling. EMBO J. 2002, 21, 6781–6790. [Google Scholar] [CrossRef] [PubMed]
- Bohgaki, M.; Matsumoto, M.; Atsumi, T.; Kondo, T.; Yasuda, S.; Horita, T.; Nakayama, K.I.; Okumura, F.; Hatakeyama, S.; Koike, T. Plasma gelsolin facilitates interaction between β2 glycoprotein i and α5β1 integrin. J. Cell. Mol. Med. 2011, 15, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Pierredon, S.; Ribaux, P.; Tille, J.C.; Petignat, P.; Cohen, M. Comparative secretome of ovarian serous carcinoma: Gelsolin in the spotlight. Oncol. Lett. 2017, 13, 4965–4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokamani, I.; Looi, M.L.; Md Ali, S.A.; Mohd Dali, A.Z.; Ahmad Annuar, M.A.; Jamal, R. Gelsolin and ceruloplasmin as potential predictive biomarkers for cervical cancer by 2d-dige proteomics analysis. Pathol. Oncol. Res. 2014, 20, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Brentnall, T.A.; Pan, S.; Cooke, K.; Moyes, K.W.; Lane, Z.; Crispin, D.A.; Goodlett, D.R.; Aebersold, R.; Bronner, M.P. Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol. Cell. Proteom. 2007, 6, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andrén-Sandberg, A.; Domellöf, L. Pancreatitis and the risk of pancreatic cancer. International pancreatitis study group. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, A.; De Corte, V.; Van Impe, K.; Bruyneel, E.; Boucherie, C.; Bracke, M.; Vandekerckhove, J.; Gettemans, J. Downregulation of gelsolin family proteins counteracts cancer cell invasion in vitro. Cancer Lett. 2007, 255, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Panis, C.; Pizzatti, L.; Bufalo, A.C.; Herrera, A.C.; Victorino, V.J.; Cecchini, R.; Abdelhay, E. Early downregulation of acute phase proteins after doxorubicin exposition in patients with breast cancer. Tumour Biol. 2016, 37, 3775–3783. [Google Scholar] [CrossRef] [PubMed]
- Jayapalan, J.J.; Lee, C.S.; Lee, C.C.; Ng, K.L.; Junit, S.M.; Hashim, O.H. Itraq analysis of urinary proteins: Potential use of gelsolin and osteopontin to distinguish benign thyroid goiter from papillary thyroid carcinoma. Clin. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wen, L.; Ma, X.; Chen, Z.; Yu, Y.; Zhu, J.; Wang, Y.; Liu, Z.; Liu, H.; Wu, D.; et al. High expression of lactotriaosylceramide, a differentiation-associated glycosphingolipid, in the bone marrow of acute myeloid leukemia patients. Glycobiology 2012, 22, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kułakowska, A.; Byfield, F.J.; Zendzian-Piotrowska, M.; Zajkowska, J.M.; Drozdowski, W.; Mroczko, B.; Janmey, P.A.; Bucki, R. Increased levels of sphingosine-1-phosphate in cerebrospinal fluid of patients diagnosed with tick-borne encephalitis. J. Neuroinflamm. 2014, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Wątek, M.; Durnaś, B.; Wollny, T.; Pasiarski, M.; Góźdź, S.; Marzec, M.; Chabowska, A.; Wolak, P.; Żendzian-Piotrowska, M.; Bucki, R. Unexpected profile of sphingolipid contents in blood and bone marrow plasma collected from patients diagnosed with acute myeloid leukemia. Lipids Health Dis. 2017, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Bhan, I.; Thadhani, R. The potential role of plasma gelsolin in dialysis-related protein-energy wasting. Blood Purif. 2010, 29, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.; Hruska, K. Osteopontin stimulates gelsolin-associated phosphoinositide levels and phosphatidylinositol triphosphate-hydroxyl kinase. Mol. Biol Cell. 1996, 7, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Tomas, A.; Yermen, B.; Min, L.; Pessin, J.E.; Halban, P.A. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: Role of gelsolin and cooperation with the mapk signalling pathway. J. Cell. Sci. 2006, 119, 2156–2167. [Google Scholar] [CrossRef] [PubMed]
- Yermen, B.; Tomas, A.; Halban, P.A. Pro-survival role of gelsolin in mouse beta-cells. Diabetes 2007, 56, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kalwat, M.A.; Wiseman, D.A.; Luo, W.; Wang, Z.; Thurmond, D.C. Gelsolin associates with the n terminus of syntaxin 4 to regulate insulin granule exocytosis. Mol. Endocrinol. 2012, 26, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; van Baelen, H.; de Moor, P. The measurement of the vitamin d-binding protein in human serum. J. Clin. Endocrinol. Metab. 1977, 45, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Peddada, N.; Dolma, K.; Khatri, N.; Ashish, A. Pregnancy related hormones, progesterone and human chorionic gonadotrophin, upregulate expression of maternal plasma gelsolin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, Y.; Zhou, Q.; Zhou, J.; Wang, H.; Jin, H.; Liu, X.; Ma, D.; Li, X. Quantitative proteomic analysis of serum from pregnant women carrying a fetus with conotruncal heart defect using isobaric tags for relative and absolute quantitation (itraq) labeling. PLoS ONE 2014, 9, e111645. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, A.K.; Kolialexi, A.; Mavrou, A.; Vougas, K.; Papantoniou, N.; Antsaklis, A.; Kanavakis, E.; Fountoulakis, M.; Tsangaris, G.T. Proteomic analysis of amniotic fluid in pregnancies with klinefelter syndrome foetuses. J. Proteom. 2010, 73, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kolla, V.; Jenö, P.; Moes, S.; Tercanli, S.; Lapaire, O.; Choolani, M.; Hahn, S. Quantitative proteomics analysis of maternal plasma in down syndrome pregnancies using isobaric tagging reagent (itraq). J. Biomed. Biotechnol 2010, 2010, 952047. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Camoin, L.; Guillonneau, F.; Rigourd, V.; Chelbi, S.T.; Leduc, M.; Laparre, J.; Mignot, T.M.; Vaiman, D. Serum profile in preeclampsia and intra-uterine growth restriction revealed by itraq technology. J. Proteom. 2010, 73, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Latest advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Whitley, G.S.; Cartwright, J.E. Pre-eclampsia: Fitting together the placental, immune and cardiovascular pieces. J. Pathol. 2010, 221, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Tannetta, D.S.; Magee, L.A.; Fuchisawa, A.; Redman, C.W.; Sargent, I.L.; von Dadelszen, P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 2006, 27, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Haverland, N.; Pottiez, G.; Wiederin, J.; Ciborowski, P. Immunoreactivity of anti-gelsolin antibodies: Implications for biomarker validation. J. Transl. Med. 2010, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Fock, U.; Jockusch, B.M.; Schubert, W.D.; Hinssen, H. Topological assignment of the n-terminal extension of plasma gelsolin to the gelsolin surface. Biochem. J. 2005, 385, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Waxman, A. The importance of differentiating gelsolin isoforms. Am. J. Respir. Crit. Care Med. 2006, 173, 685, author reply 685. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, M.; Joenväärä, S.; Seppänen, H.; Mustonen, H.; Haglund, C.; Renkonen, R. Comparative proteomic profiling of the serum differentiates pancreatic cancer from chronic pancreatitis. Cancer Med. 2017, 6, 1738–1751. [Google Scholar] [CrossRef] [PubMed]




| Disease | pGSN | Material | Detection Method | Suggested Mechanism of pGSN Changes | Ref. | |
|---|---|---|---|---|---|---|
| Trauma | Major trauma | ↓ | blood | western blot | binding of actin from damaged cells, formation of actin-gelsolin complexes | [18] |
| Critically ill patients | ↓ | blood | nucleation assay | binding of actin from damaged cells, formation of actin-gelsolin complexes | [46] | |
| Burns | ↓ | blood | ELISA | binding of actin from damaged cells, formation of actin-gelsolin complexes, binding of inflammatory mediators, proteolytic cleavage by MMPs (*) | [12,47,48] | |
| Traumatic brain injury | ↓ | blood | ELISA | actin binding, formation of actin-gelsolin complexes, binding of inflammatory mediators and diminishing of neuroinflammation | [50] | |
| CPB-ALI | ↓ | blood | ELISA | actin binding, formation of actin-gelsolin complexes, binding of inflammatory mediators | [51] | |
| Acute liver injury | ↓ | blood | ELISA | binding of actin released from injured liver | [53] | |
| Infections and infectious-associated diseases | Sepsis | ↓ | blood | ELISA, nucleation assay | binding of actin from damaged cells, formation of actin-gelsolin complexes, binding of inflammatory mediators | [17,54,55] |
| Malaria | ↓ | blood | nucleation assay, severing assay, western blot, LC/MS/MS | binding of actin released from destroyed erythrocytes, binding of hemozoin and formation of hemozoin-gelsolin complexes | [56,57] | |
| HBV-induced cirrhosis | ↓ | blood | 2-DE, MS/MS | not defined | [58] | |
| HAD | ↓ | CSF | 2-DE, 2-D DIGE, western blot | not defined | [59] | |
| ↑ | blood | SELDI-TOF | not defined | [60] | ||
| Chronic inflammatory diseases | Rheumatic arthritis | ↓ | blood, synovial fluid | nucleation assay | distribution of gelsolin into inflamed synovial joint space, binding of actin from damaged cells, formation of actin-gelsolin complexes, decreased production (*), proteolytic degradation (*), binding to plasma factors (*) | [61] |
| ↓ | urine | CE-MS | not defined | [62] | ||
| ↑ | urine | ELISA | not defined | [63] | ||
| Atopic dermatitis | ↓ | blood | ELISA | binding of inflammatory mediators, prevention of Fas-induced keratinocyte apoptosis (*) | [64] | |
| Neurological disorders | Alzheimer’s disease | ↓ | CSF | 2-D DIGE, MS, ELISA | not defined | [65] |
| TBE, LNB | ↓ | blood | western blot | binding of actin from damaged cells, formation of actin-gelsolin complexes, binding of inflammatory mediators | [66] | |
| Multiple sclerosis | ↓ | blood, CSF | western blot | binding of actin from damaged cells, binding of inflammatory mediators and diminishing of neuroinflammation | [67] | |
| SAH | ↓ | blood, CSF | ELISA, western blot | proteolytical cleavage by MMP-3, MMP-1 and MMP-9 | [68,69,70] | |
| Cancers | Colon cancer | ↑ | blood | ELISA, western blot | interaction with extracellular environment proteins, increase of colon cancer motility | [71] |
| Pancreatic cancer and pancreatitis | ↑ | blood | SRM, ELISA | not defined | [72] | |
| Breast cancer | ↓ | blood | LC-MS/MS, western blot | BRCA1-dependent recruiting of ATF-1 | [73] | |
| Astrocytoma | ↓ | CSF | 2-DE, MALDI-TOF/TOFMS | cleavage by caspase activity | [74] | |
| Other diseases | Chronic kidney disease | ↓ | blood | nucleation assay | impaired pGSN synthesis due to muscle wasting, actin-mediated increase of gelsolin clearance | [75] |
| AAC | ↓ | blood | ELISA | not defined | [76] | |
| Diabetes | ↓ | blood | LC-MS | binding of actin from damaged cells, formation of actin-gelsolin complexes, protein anabolism | [77] | |
| Diabetic retinopathy | ↓ | blood | SQ-MRM, SID-MRM | not defined | [78] | |
| Pre-eclampsia | ↓ | blood | ELISA | binding of actin from damaged cells, formation of actin-gelsolin complexes, proteolytic cleavage by MMPs | [79,80] | |
| Rhabdomyolysis | ↑ | blood | radioimmunoassay | induced synthesis, liberation of GSN from gelsolin-actin complexes | [81] | |
| FAF | ↑ | blood | nucleation assay | impaired gelsolin-actin interactions resulting from mutation in pGSN | [82] |
| Disease | Used Animals | Mechanism of Therapeutic Action of Gelsolin | Ref. |
|---|---|---|---|
| Lung injuries | Mice, rats | decrease of acute inflammatory response, binding of inflammatory mediators, limitation of neutrophil migration, inhibition of neutrophil adhesion to endothelial surface, improvement of pulmonary microvascular functions | [38,83] |
| Burns and thermal injuries | Mice | decrease of acute neuroinflammatory response, binding of inflammatory mediators, decrease of elevated caspase-3 activity, improvement of peripheral T lymphocyte functions, regulation of oxidative response, shortening of bleeding time | [42,84] |
| Sepsis | Mice, rats | binding of free circulating actin released from damaged cells, decrease of acute inflammatory response, binding of inflammatory mediators | [85,86] |
| Pneumonia | Mice | decrease of acute inflammatory response, binding of inflammatory mediators, improvement of bacterial clearance by macrophages via NOS3-dependent mechanism | [87] |
| Alzheimer’s disease | Mice | decrease of apoptosis, regulation of oxidative response, limitation of Aβ fibrillogenesis and Aβ-induced neurotoxicity | [88,89] |
| MS/EAE | Mice | decrease of acute neuroinflammatory response, binding of inflammatory mediators, regulation of oxidative response | [35] |
| Diabetes | Mice | depolymerization of F-actin | [36] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piktel, E.; Levental, I.; Durnaś, B.; Janmey, P.A.; Bucki, R. Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target. Int. J. Mol. Sci. 2018, 19, 2516. https://doi.org/10.3390/ijms19092516
Piktel E, Levental I, Durnaś B, Janmey PA, Bucki R. Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target. International Journal of Molecular Sciences. 2018; 19(9):2516. https://doi.org/10.3390/ijms19092516
Chicago/Turabian StylePiktel, Ewelina, Ilya Levental, Bonita Durnaś, Paul A. Janmey, and Robert Bucki. 2018. "Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target" International Journal of Molecular Sciences 19, no. 9: 2516. https://doi.org/10.3390/ijms19092516