1. Introduction
Dietary fiber (DF) including non-starch polysaccharides, lignin, non-digestible oligosaccharides, and resistant starch is not hydrolyzed by endogenous enzymes in the small intestine and becomes available for bacterial fermentation in the large intestine [
1]. For monogastric animals such as pigs, high fiber content in the diet was usually associated with decreased nutrient and energy digestibility [
2]. However, the negative impact of fiber on the nutrient utilization of pigs varied between different fiber sources and different fiber properties [
3,
4]. Recently, there has been increased interest in the application of DF in pig nutrition, not only for economic reasons, but also for its potential roles in promoting gut health and improving the innate immune defense. DF fermentation in the hindgut of pigs results in the production of short chain fatty acids (SCFAs), which may be utilized by the intestinal cells as energy sources or available for the growth of beneficial bacteria [
5]. In addition, various types of DF have been shown to enhance the intestinal barrier functions and ameliorate inflammatory responses, thereby promoting overall gut health in pigs [
6,
7].
Oat bran is one of the major byproducts in the processing of husked oat, which contain relatively high levels of protein, minerals, vitamins, and soluble β-glucan [
8]. Previous studies have shown that oat bran, supplemented with oil, may be useful in the diet of growing pigs [
9,
10]. It was demonstrated that oat bran can increase energy digestibility and fiber utilization of gestating sows better than wheat straw and sugar beet pulp [
3]. The DF components of oat bran that escape enzymatic digestion in the small intestine are almost entirely fermented by bacteria in the large intestine due to their water-soluble and fermentable properties [
11], and produce almost twice as many SCFAs as wheat bran [
9]. Oat bran was found to stimulate the growth of beneficial bacteria and exert a positive response on improving gut health [
12]. What’s more, a few studies reported that oat bran may reduce the oxidative stress and inflammatory responses [
13,
14]. Thus, oat bran becomes an important consideration as an alternative feed ingredient in swine production. However, continued investigations are needed to clarify the beneficial role of oat bran regarding the gut health of pigs.
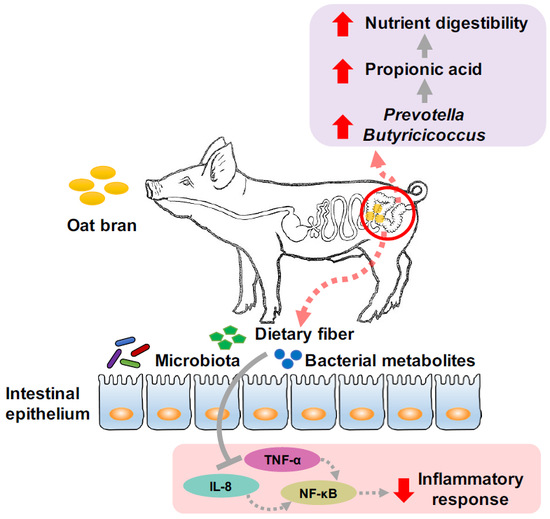
The objective of this study was to test the hypothesis that the addition of oat bran to pig diet could affect nutrient digestibility, intestinal microbiota composition, and fermentation profiles, as well as inflammatory responses in the hindgut of growing pigs.
3. Discussion
In past decades, the rapid development of swine production was accompanied by growing concerns about the economic pressure of feed cost and antibiotic resistance [
15]. Inclusion of DF in the diet was thought to be an effective way to reduce feed cost and improve the gut health of pigs. Oat bran was considered an important alternative feed ingredient in swine production. However, there is still a need for more research to understand the functional roles of oat bran in the pig gastrointestinal tract and its application in swine production. In this study, pigs were fed either a conventional corn-soybean meal diet (basal diet) or a basal diet supplemented with 10% oat bran, to determine the effect of oat bran on growth performance, nutrient digestibility, microbiota composition, and fermentation profiles, as well as the inflammatory responses in the hindgut of growing pigs.
Dietary administration of oat bran did not affect ADG or ADFI of the pigs during the experimental period compared to the basal diet treatment (
Table 1). The digestibility of dietary GE, DM, OM, and CP was lower in the OB group on day 14, compared to the CON group (
Table 2). This was consistent with other studies that reported the physico-chemical properties of oat bran, such as viscosity and water solubility, which may increase digesta viscosity and limit the interaction between nutrients and enzymes in the small intestine [
16], thereby reducing nutrient digestion and absorption. However, nutrient digestibility was not affected by the addition of oat bran after the 28 day treatment (
Table 2). DF fermentation in the large intestine can result in the production of SCFAs, and the energy produced from these metabolically important molecules may contribute up to 15% of the energy maintenance requirements of growing pigs [
17]. In our results, the concentration of propionic acid was higher in the colonic digesta of the OB group compared to the CON group (
Table 4), which may partly help improve the nutrient digestibility of the OB group. In addition, previous studies showed that intestinal bacteria will adapt and ferment complex carbohydrates more efficiently [
18,
19]. Although the nutrient digestibility was lower in the OB group compared to the CON group on day 14, the enhanced fiber fermentation and increased SCFAs production during subsequent weeks may lead to a similar nutrient digestibility in the OB group on day 28, compared to the CON group.
Fiber components in the diet are important factors that influence the intestinal bacteria in swine [
5,
12]. Using 16S rRNA sequencing, we determined the microbiota composition in the caecal and colonic digesta of the CON and OB groups. The Sobs index was lower in the caecal digesta of the OB group compared to the CON group, while the Shannon index in the colonic digesta was the same between the two groups (
Figure 1). Consistent with previous studies, the microbiota of the caecal and colonic digesta from both groups were dominated by Firmicutes and Bacteroidetes (
Figure 2) [
20]. At the genus level, the abundance of
Peptococcus was lower in the caecal digesta of the OB group, and
Catenibacterium was higher in the caecal digesta of the OB group compared to the CON group (
Table 3).
Peptococcus was frequently isolated from piggery wastes [
21], while
Catenibacterium was once discovered to have a significant increased abundance in pigs infected with
Salmonella enterica [
22,
23]. Information about these two genera, for the most part, is currently lacking and thus there exists a great need for further research of these bacteria. As to the colonic digesta, the relative abundance of
Prevotella,
Butyricicoccus, and
Catenibacterium were higher in the OB group compared to the CON group. The genus
Prevotella was found to be positively correlated with the production of SCFAs and the metabolism of amino acids, energy, cofactors, and vitamins in the host [
24]. Specifically, the presence of
Prevotella decreased in pigs suffering from post-weaning diarrhea [
25,
26].
Butyricicoccus, a butyrate-producing genera belonging to the family Ruminococcaceae, had a higher abundance in fecal samples from pigs fed whole grain barley and oat diet, compared to pigs fed the extruded cereal diet [
27]. Consistently, the concentration of propionic acid in the colonic digesta of the OB group remained higher than that of the CON group. Therefore, the colonic digesta of pigs fed an oat bran diet may be predominated with cellulolytic bacteria, such as
Prevotella and
Butyricicoccus, resulting in a higher production of SCFAs, which in turn provides a more sustained homeostatic balance leading to a heathier gut. Two other genera,
Coprococcus and
Desulfovibrio, were discovered to have a lower abundance in the colonic digesta of the OB group compared to the CON group (
Table 4). Previous studies reported that the abundance of genus
Coprococcus was significantly higher in the hindgut of pigs fed a diet containing a high level of resistant starch [
28], and several species of the genus
Coprococcus were associated with the production of butyric acid [
29]. The genus
Desulfovibrio was discovered to have a higher abundance in pigs fed a pea fiber diet with a possible connection to fiber degradation [
30]. Although
Coprococcus and
Desulfovibrio may participate in fiber digestion, the negligible portion of these bacteria in the colonic digesta of pigs may weaken their contributions, compared to other cellulolytic bacteria, such as
Prevotella. In addition, several
Desulfovibrio species were considered as significant features in identifying dysentery and intestinal dysbiosis [
31,
32], which was lower in the colonic digesta of the OB group.
The presence of DF in the hindgut affects intestinal microbial environment, leading to a possible connection to changes in intestinal functions. Oat bran and its fiber components have been well studied for their beneficial role in alleviating oxidative stress and inflammatory responses in humans [
33,
34]. In this study, the mRNA expression of IL-8 was lower in the caecum of the OB group compared to the CON group, while the mRNA expressions of IL-8, NF-κB, and TNF-α were lower in the colon of the OB group (
Figure 3). Intestinal pro-inflammatory cytokines, such as IL-8 and TNF-α, have been shown to increase intestinal permeability through the dysregulation of tight junction proteins [
35,
36]. NF-κB is an important transcription factor involved in the regulation of inflammation and immune responses [
37]. Previous studies have shown that the phenolic compounds present in oat bran have a beneficial effect on the oxidative stability of pig meat [
13], and oat bran intake effectively reduced oxidative stress induced by a high-fat diet in pigs [
14]. Therefore, decreased mRNA expressions of the inflammation factors in the OB group confirmed the functional roles of oat bran in alleviating inflammatory responses in the hindgut of growing pigs, which may greatly contribute to improved gut health. Previous studies have also concluded that DF improved the intestinal barrier functions of the ileum and colon in weaned piglets, a result which was probably mediated by changes in the microbiota composition [
6]. For example, the mRNA expression of occludin, ZO-1, ZO-2, and cingulin were upregulated by
Lactobacillus [
38], and
Escherichia coli could disassemble the tight junction structure of epithelial cells [
39]. However, in this study, the addition of oat bran did not affect the mRNA expression of ZO-1 and occludin in the caecum or colon (
Figure 3). A possible reason for this may be that the varied digestible and fermentable ability of different DF sources could have different effects on intestinal barrier functions.
In summary, oat bran inclusion at 10% in the diet had no effect on growth performance and nutrient digestibility of pigs on day 28 of the trial. Oat bran enriched the abundance of Prevotella, Butyricicoccus, and Catenibacterium in the colonic digesta. Increasing the relative abundance of these bacteria may enhance the fermentation of fiber to produce SCFAs, thereby improving gut health and nutrient utilization. In addition, oat bran decreased mRNA expression of IL-8 in the caecum and reduced IL-8, NF-κB, and TNF-α gene levels in the colon. Such results emphasize the functional roles of oat bran on ameliorating inflammatory responses in the hindgut of growing pigs.