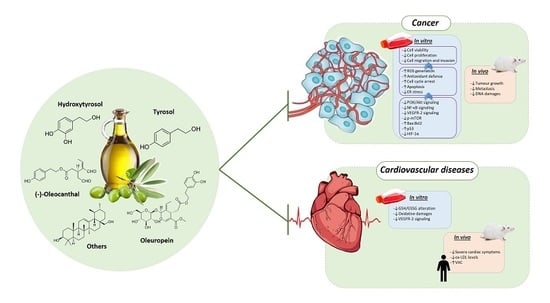
Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases
Abstract
:1. Introduction
2. Olive Oil and Phenolic Compounds
2.1. Hydroxytyrosol and Derivatives
2.2. Tyrosol
2.3. (−)-Oleocanthal
2.4. Oleuropein and Oleuropein Aglycone
2.5. Others Minor Compounds
3. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AO | oleanolic acid |
| AOM | azoxymethane |
| COX-1 | cyclooxygenase-1 |
| COX-2 | cyclooxygenase-2 |
| CSC | cancer stem cell |
| CVDs | cardiovascular diseases |
| DOA | decarboxymethyl OLE aglycone |
| EA | elenolic acid |
| ECS | endocannabinoid system |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| ET-1 | endothelin-1 |
| EVOO | extra virgin olive oil |
| FFA | free fatty acid |
| GSH | glutathione |
| GSSG | oxidized glutathione |
| HGF | hepatocyte growth factor |
| HT | hydroxytyrosol |
| HUVEC | human umbilical cord vein endothelial |
| IL-1β | interleukin 1 beta |
| IL-10 | interleukin-10 |
| iNOS | inducible nitric oxide synthase |
| Laur-HT | hydroxytyrosyl laurate |
| LPS | lipopolysaccharide |
| MD | Mediterrean Diet |
| MMP | matrix metalloproteinase |
| MMP-9 | matrix metalloproteinase 9 |
| MMP-9/NGAL | neutrophil gelatinase-associated lipocalin complex |
| NF-κB | nuclear factor-kappa B |
| NO | nitric oxide |
| OLE | oleuropein |
| p-mTOR | phosphorylated mammalian target of rapamycin |
| PI3K | phosphoinositide 3-kinase |
| ROS | reactive oxygen species |
| TNFα | tumor necrosis factor α |
| TTR | transthyretin |
| Tyr | tyrosol |
| UV | uvaol |
| VEGF | vascular endothelial growth factor |
| VEGFR-2 | vascular endothelial growth factor receptor-2 |
| VOO | virgin olive oil |
References
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Bernard, W.S.; Wilde, C.P. World Cancer Report 2015; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Lanier, J.B.; Bury, D.C.; Richardson, S.W. Diet and physical activity for cardiovascular disease prevention. Am. Fam. Phys. 2016, 93, 919–924. [Google Scholar]
- Willett, W.C.; Koplan, J.P.; Nugent, R.; Dusenbury, C.; Puska, P.; Gaziano, T.A. Prevention of chronic disease by means of diet and lifestyle changes. In Disease Control Priorities in Developing Countries; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; World Bank: Washington, DC, USA, 2006; ISBN 978-0-8213-6179-5. [Google Scholar]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO-FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation: 10–14 November 2008, Geneva; Food and Agriculture Organization of the United Nations, Ed.; FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; ISBN 978-92-5-106733-8. [Google Scholar]
- Hooper, L.; Abdelhamid, A.; Moore, H.J.; Douthwaite, W.; Skeaff, C.M.; Summerbell, C.D. Effect of reducing total fat intake on body weight: Systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ 2012, 345, e7666. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Dubnov, G.; Niaz, M.A.; Ghosh, S.; Singh, R.; Rastogi, S.S.; Manor, O.; Pella, D.; Berry, E.M. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): A randomised single-blind trial. Lancet 2002, 360, 1455–1461. [Google Scholar] [CrossRef]
- Stark, A.H.; Madar, Z. Olive oil as a functional food: Epidemiology and nutritional approaches. Nutr. Rev. 2002, 60, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Bamia, C.; Lagiou, P.; Trichopoulos, D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am. J. Clin. Nutr. 2010, 92, 620–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visioli, F.; Galli, C. The effect of minor constituents of olive oil on cardiovascular disease: New findings. Nutr. Rev. 1998, 56, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Forastiere, F.; Farchi, S.; Mallone, S.; Trequattrinni, T.; Anatra, F.; Schmid, G.; Perucci, C.A. The protective effect of the Mediterranean diet on lung cancer. Nutr. Cancer 2003, 46, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Med. 2015, 4, 1933–1947. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Spencer, J.; Dessì, M. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health 2009, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Foundation Expert Group Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Bosetti, C.; Negri, E.; Lipworth, L.; La Vecchia, C. Olive oil and cancer risk: An update of epidemiological findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Gonzalez, C.A. The role of olive oil in disease prevention: A focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. Br. J. Nutr. 2015, 113 (Suppl. 2), S94–S101. [Google Scholar] [CrossRef]
- Braga, C.; Vecchia, C.L.; Franceschi, S.; Negri, E.; Parpinel, M.; Decarli, A.; Giacosa, A.; Trichopoulos, D. Olive oil, other seasoning fats, and the risk of colorectal carcinoma. Cancer 1998, 82, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, X.Y.; Sun, S.R.; Wang, L.X.; Huang, T. Vegetable oil intake and breast cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2015, 16, 5125–5135. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Mayén, A.L.; Agudo, A.; Travier, N.; Navarro, C.; Huerta, J.M.; Chirlaque, M.D.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; et al. Olive oil intake and mortality within the Spanish population (EPIC-Spain). Am. J. Clin. Nutr. 2012, 96, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckland, G.; Travier, N.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; Sánchez, M.-J.; Molina-Montes, E.; Chirlaque, M.D.; Huerta, J.M.; Navarro, C.; et al. Olive oil intake and CHD in the European Prospective Investigation into Cancer and Nutrition Spanish cohort. Br. J. Nutr. 2012, 108, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, B.; Masala, G.; Saieva, C.; Salvini, S.; Calonico, C.; Sacerdote, C.; Agnoli, C.; Grioni, S.; Frasca, G.; Mattiello, A.; et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: The EPICOR Study. Am. J. Clin. Nutr. 2011, 93, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Dominguez, L.J.; Delgado-Rodríguez, M. Olive oil consumption and risk of CHD and/or stroke: A meta-analysis of case-control, cohort and intervention studies. Br. J. Nutr. 2014, 112, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Nascetti, S.; López-Sabater, M.C.; Elosua, R.; Salonen, J.T.; Nyyssönen, K.; Poulsen, H.E.; Zunft, H.-J.F.; Kiesewetter, H.; de la Torre, K.; et al. EUROLIVE Study Group Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: The EUROLIVE study. J. Am. Coll. Nutr. 2008, 27, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.-I.; Konstantinidou, V.; Fitó, M. Olive Oil and Cardiovascular Health. J. Cardiovasc. Pharmacol. 2009, 54, 477–482. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Carbot, K.; Chávez-Servín, J.L.; Jaúregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Nurmi, T.; Poulsen, H.E.; Gaddi, A.V.; Kaikkonen, J.; Zunft, H.-F.; et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J. Nutr. 2010, 140, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Mataix, J.; Quiles, J.L.; Huertas, J.R.; Battino, M.; Mañas, M. Tissue specific interactions of exercise, dietary fatty acids, and vitamin E in lipid peroxidation. Free Radic. Biol. Med. 1998, 24, 511–521. [Google Scholar] [CrossRef]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Guccione, G.D.; Accardi, G.; Caruso, C. What olive oil for healthy ageing? Maturitas 2015, 80, 117–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation effects on quality, phenolic composition, and selected volatiles of virgin olive oils cv. Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU) 2015/1833 of 12 October 2015 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2015.266.01.0029.01.ENG&toc=OJ:L:2015:266:TOC (accessed on 1 July 2018).
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, Ó.; Díaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Biological properties of olive oil phytochemicals. Crit. Rev. Food Sci. Nutr. 2002, 42, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Nicolaïew, N.; Lemort, N.; Adorni, L.; Berra, B.; Montorfano, G.; Rapelli, S.; Cortesi, N.; Jacotot, B. Comparison between extra virgin olive oil and oleic acid rich sunflower oil: Effects on postprandial lipemia and LDL susceptibility to oxidation. Ann. Nutr. Metab. 1998, 42, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Galli, C.; Visioli, F. Antioxidant and other activities of phenolics in olives/olive oil, typical components of the Mediterranean diet. Lipids 1999, 34, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Bilancia, M.T.; Pasqualone, A.; Sikorska, E.; Gomes, T. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur. Food Res. Technol. 2005, 221, 92–98. [Google Scholar] [CrossRef]
- Caponio, F.; Squeo, G.; Brunetti, L.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Catalano, P.; Bianchi, B. Influence of the feed pipe position of an industrial scale two-phase decanter on extraction efficiency and chemical-sensory characteristics of virgin olive oil. J. Sci. Food Agric. 2018, 98, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Squeo, G.; Tamborrino, A.; Pasqualone, A.; Leone, A.; Paradiso, V.M.; Summo, C.; Caponio, F. Assessment of the Influence of the Decanter Set-up During Continuous Processing of Olives at Different Pigmentation Index. Food Bioprocesss Technol. 2017, 10, 592–602. [Google Scholar] [CrossRef]
- Squeo, G.; Silletti, R.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Influence of calcium carbonate on extraction yield and quality of extra virgin oil from olive (Olea europaea L. cv. Coratina). Food Chem. 2016, 209, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escribá, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, S.; Blades, M. The Mediterranean diet—A review of evidence relevant to the food and drink industry. Nutr. Food Sci. 2013, 43, 7–16. [Google Scholar] [CrossRef]
- Makedou, K.; Papandreou, D.; Karampola, M. The role of Mediterranean diet in health and disease: An updated mini review. Nutr. Food Sci. 2011, 41, 63–72. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergens. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), ‘‘anti-inflammatory properties’’ (ID 1882), ‘‘contributes to the upper respiratory tract health’’ (ID 3468), ‘‘can help to maintain a normal function of gastrointestinal tract’’(3779), and ‘‘contributes to body defenses against external agents’’ (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033–2058. [Google Scholar]
- Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0432 (accessed on 1 July 2018).
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Marinelli, N.; Scozzafava, G.; Romano, C.; Contini, C.; Casini, L. Nutraceutical olive oil: Does it make the difference? Nutr. Food Sci. 2014, 44, 586–600. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef] [Green Version]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Keceli, T.M.; Kamiloglu, S.; Capanoglu, E. Phenolic Compounds of Olives and Olive Oil and their Bioavailability. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Shahidi, F., Kiritsakis, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; Chapter 24; pp. 457–470. [Google Scholar]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Weinbrenner, T.; Fito, M.; Farre, A.M.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; De la Torre, R.; Covas, M.I. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Exp. Clin. Res. 2003, 30, 207–212. [Google Scholar]
- Covas, M.I.; De la Torre, K.; Farre-Albaladejo, M.; Kaikkonen, J.; Fito, M.; Lopez-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventos, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.I.; Farre, M.; Fito, M.; Ortuno, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Tsimidou, M. Polyphenols and Quality of Virgin Olive Oil in Retrospect. Ital. J. Food Sci. 1998, 10, 99–116. [Google Scholar]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1991, 58, 966–968. [Google Scholar] [CrossRef]
- Krichene, D.; Salvador, M.D.; Fregapane, G. Stability of Virgin Olive Oil Phenolic Compounds during Long-Term Storage (18 Months) at Temperatures of 5–50 °C. J. Agric. Food Chem. 2015, 63, 6779–6786. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Z.; Ma, Y.; Song, R.; Pei, T.; Zheng, T.; Wang, J.; Xu, D.; Fang, X.; Jiang, H.; et al. Hydroxytyrosol inhibits cholangiocarcinoma tumor growth: An in vivo and in vitro study. Oncol. Rep. 2014, 31, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, P.; Fuccelli, R.; Sepporta, M.V.; Fabiani, R. In vitro chemo-preventive activities of hydroxytyrosol: The main phenolic compound present in extra-virgin olive oil. Food Funct. 2016, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162. [Google Scholar] [CrossRef] [PubMed]
- López de Las Hazas, M.-C.; Piñol, C.; Macià, A.; Motilva, M.-J. Hydroxytyrosol and the Colonic Metabolites Derived from Virgin Olive Oil Intake Induce Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Ouanouki, A.; Béliveau, R.; Desrosiers, R.R. Olive oil compounds inhibit vascular endothelial growth factor receptor-2 phosphorylation. Exp. Cell Res. 2014, 322, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.; Camacho-Corencia, P.; Sanchez-Rovira, P.; Vera-Ramirez, L.; Ramirez-Tortosa, M. Hydroxytyrosol inhibits growth and cell proliferation and promotes high expression of sfrp4 in rat mammary tumours. Mol. Nutr. Food Res. 2011, 55, S117–S126. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; Micioni Di Bonaventura, M.V.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Geng, C.; Jiang, L.; Cao, J.; Yoshimura, H.; Zhong, L. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother. Res. 2009, 23, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Dávalos, A.; Visioli, F. Chronic hydroxytyrosol feeding modulates glutathione-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Roselló-Catafau, J.; Pintó, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014, 2, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Martínez-López, S.; Baeza Arévalo, G.; Amigo-Benavent, M.; Sarriá, B.; Bravo-Clemente, L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016, 205, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Dangles, O.; Rakotomanomana, N.; Baracchini, S.; Visioli, F. 3-O-Hydroxytyrosol glucuronide and 4-O-hydroxytyrosol glucuronide reduce endoplasmic reticulum stress in vitro. Food Funct. 2015, 6, 3275–3281. [Google Scholar] [CrossRef] [PubMed]
- Ruano, J.; López-Miranda, J.; de la Torre, R.; Delgado-Lista, J.; Fernández, J.; Caballero, J.; Covas, M.I.; Jiménez, Y.; Pérez-Martínez, P.; Marín, C.; et al. Intake of phenol-rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients. Am. J. Clin. Nutr. 2007, 86, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Kitakaze, M. ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Bernardini, E. Extra virgin olive oil’s polyphenols: Biological activities. Curr. Pharm. Des. 2011, 17, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Miró-Casas, E.; Covas, M.-I.; Fitó, M.; Farré-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Han, Q.; Breslin, P.A.S.; Beauchamp, G.K. Synthesis and assignment of absolute configuration of (-)-oleocanthal: A potent, naturally occurring non-steroidal anti-inflammatory and anti-oxidant agent derived from extra virgin olive oils. Org. Lett. 2005, 7, 5075–5078. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Gómez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gómez-Reino, J.J.; Smith, A.B.; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Medina, E.; Vargas, J.; Brenes, M.; De Castro, A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2007, 55, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; Sayed, K.A.E. Olive Phenolics as c-Met Inhibitors: (−)-Oleocanthal Attenuates Cell Proliferation, Invasiveness, and Tumor Growth in Breast Cancer Models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef] [PubMed]
- LeGendre, O.; Breslin, P.A.; Foster, D.A. (−)-Oleocanthal rapidly and selectively induces cancer cell death via lysosomal membrane permeabilization. Mol. Cell. Oncol. 2015, 2, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Olive Oil-derived Oleocanthal as Potent Inhibitor of Mammalian Target of Rapamycin: Biological Evaluation and Molecular Modeling Studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (-)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Fabiani, R. Oleuropein Prevents Azoxymethane-Induced Colon Crypt Dysplasia and Leukocytes DNA Damage in A/J Mice. J. Med. Food 2016, 19, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.K.; Elamin, M.H.; Omer, S.A.; Daghestani, M.H.; Al-Olayan, E.S.; Elobeid, M.A.; Virk, P. Oleuropein induces apoptosis via the p53 pathway in breast cancer cells. Asian Pac. J. Cancer Prev. 2014, 14, 6739–6742. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; Alarcón de la Lastra, C. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-M.; Chai, E.-Q.; Cai, H.-Y.; Miao, G.-Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, M.; Nosi, D.; Natalello, A.; Porcari, R.; Ramazzotti, M.; Chiti, F.; Bellotti, V.; Doglia, S.M.; Stefani, M.; Bucciantini, M. The polyphenol Oleuropein aglycone hinders the growth of toxic transthyretin amyloid assemblies. J. Nutr. Biochem. 2016, 30, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Morittu, V.M.; Procopio, A.; Britti, D.; Cuzzocrea, S. The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clin. Nutr. 2011, 30, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Galli, G.V.; Maschi, O.; Gilardi, F.; Bellosta, S.; Crestani, M.; Bosisio, E.; De Fabiani, E.; Caruso, D. Olive oil phenols modulate the expression of metalloproteinase 9 in THP-1 cells by acting on nuclear factor-kappaB signaling. J. Agric. Food Chem. 2010, 58, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. Oleanolic Acid, a Compound Present in Grapes and Olives, Protects against Genotoxicity in Human Mammary Epithelial Cells. Molecules 2015, 20, 13670–13688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. The differential localization of a methyl group confers a different anti-breast cancer activity to two triterpenes present in olives. Food Funct. 2015, 6, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Faja, B.; Cuyàs, E.; Lozano-Sánchez, J.; Cufí, S.; Verdura, S.; Fernández-Arroyo, S.; Borrás-Linares, I.; Martin-Castillo, B.; Martin, Á.G.; Lupu, R.; et al. Extra-virgin olive oil contains a metabolo-epigenetic inhibitor of cancer stem cells. Carcinogenesis 2018, 39, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Campos, M.; Allouche, Y.; Sánchez-Quesada, C.; Ruiz-Mora, J.; Beltrán, G.; Gaforio, J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010, 48, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Nho, C.W.; Kwon, D.Y.; Kang, Y.-H.; Lee, K.W.; Park, J.H.Y. Maslinic acid inhibits the metastatic capacity of DU145 human prostate cancer cells: Possible mediation via hypoxia-inducible factor-1α signalling. Br. J. Nutr. 2013, 109, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Han, L.; Lin, X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J. Appl. Toxicol. 2013, 33, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A.; Peragón, J. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteom. 2013, 83, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-García, F.; Dopazo, J.; Lupiáñez, J.A.; Günther, U.; Cascante, M. Maslinic acid-enriched diet decreases intestinal tumorigenesis in Apc(Min/+) mice through transcriptomic and metabolomic reprogramming. PLoS ONE 2013, 8, e59392. [Google Scholar] [CrossRef] [PubMed]
- Es-Saady, D.; Najid, A.; Simon, A.; Denizot, Y.; Chulia, A.J.; Delage, C. Effects of ursolic acid and its analogues on soybean 15-Lipoxygenase Activity and the proliferation rate of a human gastric tumour cell line. Mediat. Inflamm. 1994, 3, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Erythrodiol, a natural triterpenoid from olives, has antiproliferative and apoptotic activity in HT-29 human adenocarcinoma cells. Mol. Nutr. Food Res. 2008, 52, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Ibeas, E.; Carvalho-Tavares, J.; Hernández, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Natural triterpenic diols promote apoptosis in astrocytoma cells through ROS-mediated mitochondrial depolarization and JNK activation. PLoS ONE 2009, 4, e5975. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Treatments | Main Results | Ref. |
|---|---|---|---|
| CRC adenocarcinoma cells (DLD1) | HT (0–300 µM for 24 and 48 h) | HT induces ROS generation and leads to PI3K/Akt pathway activation, decreasing the antioxidant defense capacity through FOXO3a suppression. | [71] |
| Human CCA (TFK-1 and KMBC) and human gallbladder (GBS-SD) cancer cells | HT (0–200 μM for 24, 48 and 72 h) | HT induces cell cycle arrest and apoptosis. | [72] |
| Human hepatocellular carcinoma (HepG2, Hep3B, SK-HEP-1 and Huh-7) cells | HT (0–400 µM for 48 and 72 h) | HT can suppress the activation of Akt and NF-κB pathways. | [73] |
| Human breast (MDA and MCF-7), prostate (LNCap and PC3) and colon (SW480 and HCT116) cancer cells | HT (100 µM for 24, 48, 72, 96, 120 and 144 h) | HT inhibits the proliferation of all cell lines. | [74] |
| Human thyroid carcinoma (TPC-1 and FB-2), papillary and follicular (WRO) cells | HT (65–973 μM for 24 and 48 h) | HT reduces viability in all cell lines and exerts proapoptotic effects on papillary and follicular cancer cells. | [75] |
| Human colon cancer cells (Caco-2 and HT-29) | HT (100–200 μM for 8 and 48 h) | HT produces cell cycle arrest and promotes apoptosis. | [76] |
| Human umbilical vein endothelial cells (HUVECs) and dermal microvascular endothelial cells (HMVECs-d-Ad) | HT (0–50 μM for 18 and 24 h) | HT inhibits VEGFR-2 signaling pathway. | [77] |
| Male nude BALB/c mice cholangiocarcinoma xenograft (6–8 weeks old) | Intraperitoneally injected HT (500 mg/kg, daily, 3 weeks after the tumor volume reached ~120 mm3) | HT inhibits cholangiocarcinoma growth. | [72] |
| Orthotopic HCC model in nude mice cholangiocarcinoma xenograf (4–6 weeks old) | Intraperitoneally injected HT (10 mg/kg or 20 mg/kg, daily, 3 weeks starting 14 d after inoculation) | HT inhibits cholangiocarcinoma growth. | [73] |
| Human colon cancer (Caco-2) cells | HT (50 μM for 24 h) | HT up-regulates CNR1 gene via epigenetic regulation (decrease in methylation at CNR1 promoter), which is associated with reduced proliferation of Caco-2 cells. | [79] |
| Murine pre-adipocytes (3T3-L1) exposed to H2O2 | Pretreatment with HT (1 and 5 µM for 24 h) | HT blunts the H2O2-induced GSH/GSSG alteration. | [84] |
| Human umbilical cord vein endothelial cells (HUVEC) | HT, Tyr, and combination of both (10 µM for 30 min or 18 h) | The combination of HT with Tyr preserves cell functions from oxidative damage, which correlates with rescuing their antioxidant properties. | [85] |
| Human myelomonocytic cells (U937) and murine skeletal myoblasts (C2C12) exposed to H2O2 | Pretreatment with Laur-HT (5 µM), HT (20 µM) or both combined (20 µM) (for 30 min) | Laur-HyT has a protective antioxidant effect against H2O2 treatment, greater than HyT, so having a role in the prevention of apoptotic death in normal and tumor cells. | [86] |
| Human endothelial cells (ECV304) incubated with high glucose (30 mM) in the presence or absence of 0–120 mM FFAs (oleic or linoleic acid) | Co-treatment with HT (10 µM for 48 h) and polyphenol extract from EVOO (10 µM gallic acid equivalents for 48 h) | Treatments reduce the oxidative stress and modulate changes in NO and ET-1 associated with experimental conditions that simulate diabetes (hyperglycemia and a high level of FFA). | [87] |
| Human peripheral blood mononuclear cells (PBMC) and U937 monocytes activated with PMA (30 nM) | HT (1–10 μM for 0–24 h) prior to activation with PMA | HT blunts monocyte matrix invasive potential, reduces MMP-9 release and expression, and inhibits PGE2 production and COX-2 expression, which are mediated by inhibition of NF-κB transcription, PKCα and β1 activation. | [83] |
| Healthy subjects (22–37 years) | HT-enriched biscuits (30 g that contained 5.25 mg of HT) or Non-enriched biscuits (30 g) after overnight-fasting, only one meal in a cross-overdesing | Enriched biscuits consumption leads to a peak of posprandial levels of plasma metabolites (mainly 3,4-dihydroxyphenylacetic acid (DOPAC)-sulphate, DOPAC, HVA) between 0.5 and 1 h, which are also extensively excreted in urine and lower postprandial ox-LDL levels. | [88] |
| Volunteers with mild hyperlipidemia | HT purified (99.5%) from olive mill waste (5 mg, daily, for 8 weeks) | HT does not influence markers of CVD, blood lipids, inflammatory markers, liver or kidney functions and the electrolyte balance, but increased vitamin C levels. | [89] |
| Human hepatocarcinoma (HepG2) cells under tunicamycin-induced ER stress | HT or hepatic HT-derived metabolites 3-O-HT glucuronide and 4-O-HT glucuronide (10 and 25 μM for 24 h) prior to tunicamycin treatment | Both metabolites glucuronide inhibit ER stress, although they induce a milder change in mRNA expression levels of both CHOP and BiP. | [90] |
| Sample | Treatments | Main Results | Ref. |
|---|---|---|---|
| Human breast cancer cells (MDA-MB-231, MCF-7 and BT-474) | (−)-Oleocanthal (10–100 ng/mL for 24, 48 and 72 h) | (−)-Oleocanthal inhibits growth and causes a dose-dependent inhibition of HGF-induced cell migration, invasion and G1/S cell cycle progression. | [100] |
| Human pancreatic (BxPC3), prostate (PC3) and breast (MDA-MB-231) cancer cells | (−)-Oleocanthal (0.2–20 µM for 4, 24, 48 and 72 h) | (−)-Oleocanthal induces cell death, primary necrotic and apoptotic cell death via induction of lysosomal membrane permeabilization. | [102] |
| Human breast cancer (MCF-7, T47D) metastatic breast cancer (MDA-MB-2318), CRC (Caco-2) and adenocarcinoma (HeLa) cells | (−)-Oleocanthal (10 μM for 72 h on MDA-MB-231) | (−)-Oleocanthal shows anti-proliferative against several breast cancer cell lines and down-regulates the levels of p-mTOR in the metastatic breast cancer cell line (MDA-MB-231). | [103] |
| Human hepatocellular cell lines (Huh-7, HepG2 and HCCLM3) | (−)-Oleocanthal (0–80 µM for 12, 24, 48 and 72 h) | (−)-Oleocanthal inhibits human hepatocellular carcinoma by inactivating STAT3. | [101] |
| Human breast cancer cells (BT-474, MCF-7 and T-47D) | (−)-Oleocanthal (5–60 µM for 48 h in BT-474 and MCF-7 cells; 10–100 µM for 24 and 48 h in T-47D cells) | (−)-Oleocanthal suppresses growth of all cancer cells, in part, by reducing total levels of ERα. | [104] |
| Female athymic nude Foxn1nu/Foxn1+ mice (4–5 weeks old) in human tumor xenograft model | Intraperitoneally injected (−)-oleocanthal (5 mg/kg, 3 d/week, 33 d starting 5 d after inoculation) | (−)-Oleocanthal suppresses tumor growth. | [100] |
| BALB/c athymic nude mice a in vivo human lung metastasis model hepatocellular (4–6 weeks old, male) | Intraperitoneally injected (−)-oleocanthal (5 mg/kg or 10 mg/kg, daily, 5 weeks) | (−)-Oleocanthal suppresses hepatocellular tumor growth and impedes carcinoma metastasis in lung by inactivating STAT3. | [101] |
| Female thymic nudeFoxn1nu/Foxn1+ mice (4–5 weeks old) inoculated with BT-474 cells | Intraperitoneally injected (−)-oleocanthal (5 mg/kg per d or 10 mg/kg, 3 d/week, 43 d) | (−)-Oleocanthal reduces total levels of estrogen receptors in BT-474 cells. | [104] |
| Sample | Treatments | Main Results | Ref. |
|---|---|---|---|
| Human umbilical vein endothelial cells (HUVECs) and dermal microvascular endothelial cells (HMVECs-d-Ad) | OLE (0–50 μM for 18 and 24 h) | OLE does not inhibit VEGFR-2 signaling pathway. | [77] |
| Mice with colon cancer induced by AOM injections (10 mg/kg, 1 d/week for 6 weeks) | Basal diet either enriched or not with OLE (125 mg/kg), (7 or 17 weeks) | OLE-enriched diet prevents the preneoplastic lesions in different colon segments, reducing the severity of crypt dysplasia and DNA damage in peripheral leukocytes. | [105] |
| Mouse atrial myocytes (HL-1) | OLE-aglycone (60 μM for 24 h) | Data suggest a possible use of OLE-aglycone to treat human transthyretin (TTR)-related pathologies with the aim to relieve or to delay the occurrence of the most severe cardiac symptoms. | [110] |
| Luminal MCF-7 breast cancer cell | OLE (100 μM or 200 μM for 72 h) | OLE-induced apoptosis, which is associated with Bax gene expression up-regulation and Bcl2 gene expression down-regulation via p53 pathway activation. | [106] |
| Thyroid tumorTPC-1 and BCPAP cells | OLE and Ac-OLE (10, 50, and 100 mM for 48 h) | Both treatments inhibit cell proliferation, and decrease H2O2-induced ROS levels, and p-Akt and p-ERK levels. Thus, it exerts antioxidant and inhibitory effects on growth-promoting signal pathways. | [107] |
| Human colon adenocarcinoma (HT-29) cells | OLE (0 μM–800 μM for 24, 48 and 72 h) | OLE inhibits cell growth and induces apoptosis, which is associated with a decrease in HIF-1α protein and an increase p53, but not to changes in IkB-α and MAPK cascade proteins. | [108] |
| Hepatocellular carcinoma (Huh7) and human hepatoma (HepG2) cells | OLE (0, 20, 40, 60, 80 or 100 μM for 24 h) | OLE induces apoptosis in HepG2 cells in a dose-dependent manner, via caspase activation which is mediated by changes in proapoptotic Bcl-2 family members, (BAX and Bcl-2) levels, down-regulation of PI3K/AKT signaling pathway, and ROS production increases. | [109] |
| Sample | Treatments | Main Results | Ref. |
|---|---|---|---|
| Human umbilical vein endothelial cells (HUVECs) and dermal microvascular endothelial cells (HMVECs-d-Ad) | Taxifolin (0–50 μM for 18 and 24 h) | Taxifolin inhibits VEGFR-2 signaling pathway. | [77] |
| Human breast cancer cells (MDA-MB-231 and MCF7) | AO and MA (0.001–100 μM for 4, 24, 48 and 72 h) | AO inhibits the proliferation and increases the oxidative stress of highly invasive cells. | [114] |
| Invasive human breast cancer cells (MDA-MB-231) | UV and ER (0. 001–100 µM for 4, 24, 48 and 72 h) | UV protects DNA from damage, whereas ER enhances damage to DNA. | [115] |
| SUM-159 cells subcutaneously injected into athymic nude mice; or into the 2nd right mammary fat pad of female SCID/Beige mice | Pretreatment with DOA (10, 20 μmol/L for 3 d); or graded concentrations of DOA (for 2 h) | DOA blocks the formation of multicellular tumorspheres generated from single-founder stem-like cells in a panel of genetically diverse breast cancer models and suppresses CSC-like states responsible for maintaining tumor initiating cell properties within breast cancer populations. | [116] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reboredo-Rodríguez, P.; Varela-López, A.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 2305. https://doi.org/10.3390/ijms19082305
Reboredo-Rodríguez P, Varela-López A, Forbes-Hernández TY, Gasparrini M, Afrin S, Cianciosi D, Zhang J, Manna PP, Bompadre S, Quiles JL, et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. International Journal of Molecular Sciences. 2018; 19(8):2305. https://doi.org/10.3390/ijms19082305
Chicago/Turabian StyleReboredo-Rodríguez, Patricia, Alfonso Varela-López, Tamara Y. Forbes-Hernández, Massimiliano Gasparrini, Sadia Afrin, Danila Cianciosi, Jiaojiao Zhang, Piera Pia Manna, Stefano Bompadre, José L. Quiles, and et al. 2018. "Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases" International Journal of Molecular Sciences 19, no. 8: 2305. https://doi.org/10.3390/ijms19082305