The “Magnesium Sacrifice” Strategy Enables PMMA Bone Cement Partial Biodegradability and Osseointegration Potential
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterizations of PMMA–Mg Bone Cements
2.2. Curing Characteristics
2.3. In Vitro Degradation Tests
2.4. Cell Culture
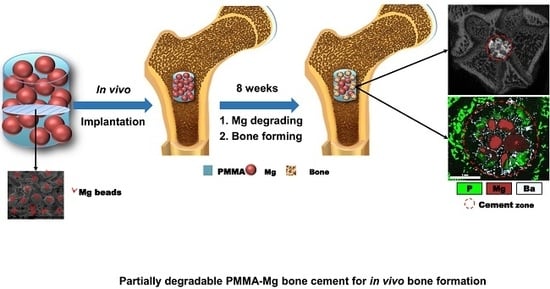
2.5. Animal Tests of PMMA–Mg Cements
3. Discussion
4. Materials and Methods
4.1. Preparation of Partially Degradable PMMA–Mg Composite Bone Cements
4.2. Characterizations of PMMA–Mg Bone Cements
4.3. Vertebral Body Augmentation Tests
4.4. Curing Characteristics
4.5. In Vitro Degradation of Bone Cement
4.6. Cell Culture
4.6.1. Cell Attachment Experiment
4.6.2. Cell Proliferation Assay
4.7. Animal Studies
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BBC | Bioactive bone cement |
| BMP-2 | Bone morphogenetic protein-2 |
| BPO | Benzoyl peroxide |
| CaP | Calcium phosphate |
| CCK-8 | Cell counting kit-8 |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| EDX | Energy dispersive X-ray spectroscopy |
| DMPT | N,N-Dimethyl-p-toluidine |
| MC | Mineralized collagen |
| Mg | Magnesium |
| MG-63 | MG-63 cells |
| MgS | Magnesium sacrifice |
| MMA | Methyl methacrylate |
| Micro-CT | Micro-focus computed tomography |
| PBS | Phosphate-buffered saline |
| PMMA | Poly (methyl methacrylate) |
| ROI | Region of interest |
| SBF | Simulated body fluid |
| SD | Sprague–Dawley (SD) rat |
| SEM | Scanning electron microscopy |
| VEGF | Vascular endothelial growth factor |
References
- Charnley, J. Anchorage of the femoral head prosthesis to the shaft of the femur. J. Bone Joint Surg. Br. 1960, 42, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.W.; Schroeder, G.D.; Anderson, P.A. Vertebroplasty and kyphoplasty for the treatment of osteoporotic vertebral compression fractures. J. Am. Acad. Orthop. Surg. 2014, 22, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Gladman, A.; Celestine, A.D.N.; Sottos, N.R.; White, S.R. Autonomic healing of acrylic bone cement. Adv. Healthc. Mater. 2015, 4, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, J.; Li, F.; Pan, Z.; Ni, X.; Shen, Y.; Xu, H.; Huang, Q. Bioactive calcium sulfate/magnesium phosphate cement for bone substitute applications. Mater. Sci. Eng. C Mater. 2014, 35, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.J.; El Othmani, M.M.; Tzeng, T.H.; Mihalko, W.M.; Chambers, M.C.; Grupp, T.M. Acrylic bone cement in total joint arthroplasty: A review. J. Orthop. Res. 2016, 34, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Sadoghi, P.; Liebensteiner, M.; Agreiter, M.; Leithner, A.; Böhler, N.; Labek, G. Revision surgery after total joint arthroplasty: A complication-based analysis using worldwide arthroplasty registers. J. Arthroplasty. 2013, 28, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Hong, S.-J.; Lee, S.; Cha, I.H.; Kim, B.-H.; Kang, E.-Y. Analysis of adjacent fracture after percutaneous vertebroplasty: Does intradiscal cement leakage really increase the risk of adjacent vertebral fracture? Skeletal Radiol. 2011, 40, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Kim, Y.J.; Yoon, T.L.; Park, S.A.; Cho, I.H.; Kim, E.J.; Kim, I.A.; Shin, J.-W. The characteristics of a hydroxyapatite–chitosan–PMMA bone cement. Biomaterials 2004, 25, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Tamura, J.; Shinzato, S.; Fujibayashi, S.; Hashimoto, M.; Kawashita, M.; Kokubo, T.; Nakamura, T. Bioactive bone cements containing nano-sized titania particles for use as bone substitutes. Biomaterials 2005, 26, 6496–6505. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, C.; Goto, K.; Imamura, M.; Nakamura, T. Bioactive bone cement with a low content of titania particles without postsilanization: Effect of filler content on osteoconductivity, mechanical properties, and handling characteristics. J. Biomed. Mater. Res. B. Appl. Biomater. 2010, 95, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical properties and cytocompatibility improvement of vertebroplasty pmma bone cements by incorporating mineralized collagen. Materials 2015, 8, 2616–2634. [Google Scholar] [CrossRef]
- Li, T.; Weng, X.; Bian, Y.; Zhou, L.; Cui, F.; Qiu, Z. Influence of nano-HA coated bone collagen to acrylic (polymethylmethacrylate) bone cement on mechanical properties and bioactivity. PLoS ONE 2015, 10, e0129018. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.; Yang, F.; Leeuwenburgh, S.C.; Wolke, J.G.; Ye, G.; de Wijn, J.R.; Jansen, J.A.; Wang, Y. Physicochemical properties and in vitro mineralization of porous polymethylmethacrylate cement loaded with calcium phosphate particles. J. Biomed. Mater. Res. B. Appl. Biomater. 2015, 103, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Boger, A.; Bohner, M.; Heini, P.; Verrier, S.; Schneider, E. Properties of an injectable low modulus PMMA bone cement for osteoporotic bone. J. Biomed. Mater. Res. B. Appl. Biomater. 2008, 86, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, Y.; Yang, J.; Tan, L.; Yang, K. Potential antiosteoporosis effect of biodegradable magnesium implanted in STZ-induced diabetic rats. J. Biomed. Mater. Res. A. 2011, 99, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Han, P.; Zhao, C.; Zhang, S.; Wu, H.; Ni, J.; Hou, P.; Zhang, Y.; Liu, J.; Xu, H. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials 2016, 81, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, L.; Liu, C.; Yuan, Y.; Lin, X.; Tan, L.; Chen, S.; Yang, K.; Mei, X. Effect of implantation of biodegradable magnesium alloy on BMP-2 expression in bone of ovariectomized osteoporosis rats. Mater. Sci. Eng. C Mater. 2013, 33, 4470–4474. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca–P coatings on biodegradable Mg alloy: In vitro biomineralization behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Thomas, K.A.; Delton, J.E.; Volkman, T.K.; Whitecloud, T.S.; Key, J.F. Hydroxylapatite coating of porous implants improves bone ingrowth and interface attachment strength. J. Biomed. Mater. Res. 1992, 26, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C Mater. 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Endogan, T.; Kiziltay, A.; Kose, G.T.; Comunoglu, N.; Beyzadeoglu, T.; Hasirci, N. Acrylic bone cements: Effects of the poly (methyl methacrylate) powder size and chitosan addition on their properties. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Zderic, I.; Steinmetz, P.; Benneker, L.M.; Röhrle, O.; Windolf, M.; Boger, A.; Gueorguiev, B. Bone cement allocation analysis in artificial cancellous bone structures. J. Orthop. Transl. 2017, 8, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Mjoberg, B.; Pettersson, H.; Rosenqvist, R.; Rydholm, A. Bone cement, thermal injury and the radiolucent zone. Acta Orthop. Scand. 1984, 55, 597–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanczyk, M.; Van Rietbergen, B. Thermal analysis of bone cement polymerisation at the cement-bone interface. J. Biomech. 2004, 37, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Chen, H.; Huang, L.; Dong, J.; Guo, D.; Mao, M.; Kong, L.; Li, Y.; Wu, Z.; Lei, W. Porous surface modified bioactive bone cement for enhanced bone bonding. PLoS ONE 2012, 7, e42525. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yeh, H.; Tian, T.; Lee, W. Degradation of magnesium alloys in biological solutions and reduced phenotypic expression of endothelial cell grown on these alloys. In Proceedings of the 3rd Kuala Lumpur International Conference on Biomedical Engineering 2006, Berlin, Germany, 11–14 December 2007; pp. 98–101. [Google Scholar]
- Seal, C.K.; Vince, K.; Hodgson, M. Biodegradable surgical implants based on magnesium alloys–A review of current research. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Auckland, New Zealand, 8–9 April 2009; p. 012011. [Google Scholar]
- Cimatti, B.; Engel, E.E.; Nogueira-Barbosa, M.H.; Frighetto, P.D.; Volpon, J.B. Physical and mechanical characterization of a porous cement for metaphyseal bone repair. Acta Ortop. Bras. 2015, 23, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-M.; Kang, M.-H.; Kim, H.-E.; Lim, H.-K.; Byun, S.-H.; Lee, J.-H.; Lee, S.-M. Innovative micro-textured hydroxyapatite and poly (l-lactic)-acid polymer composite film as a flexible, corrosion resistant, biocompatible, and bioactive coating for Mg implants. Mater. Sci. Eng. C Mater. 2017, 81, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lampin, M.; Warocquierclerout, R.; Legris, C.; Degrange, M.; Sigotluizard, M. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 1997, 36, 99–108. [Google Scholar] [CrossRef]
- Cimatti, B.; Santos, M.A.D.; Brassesco, M.S.; Okano, L.T.; Barboza, W.M.; Nogueira-Barbosa, M.H.; Engel, E.E. Safety, osseointegration, and bone ingrowth analysis of PMMA-based porous cement on animal metaphyseal bone defect model. J. Biomed. Mater. Res. B. Appl. Biomater. 2018, 106, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.; Yu, N.; Wolke, J.G.; Chanchareonsook, N.; Goh, B.T.; Wang, Y.; Yang, F.; Jansen, J.A. Bone Response to Porous Poly (methyl methacrylate) Cement Loaded with Hydroxyapatite Particles in a Rabbit Mandibular Model. Tissue Eng. Part C Methods 2017, 23, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Kin, A.; Yazu, M.; Abe, M. Biomechanical evaluation of kyphoplasty and vertebroplasty with calcium phosphate cement in a simulated osteoporotic compression fracture. J. Orthop. Sci. 2003, 8, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]










| Group | Mg Content (wt %) | Liquid Composition (g) | Solid Composition (g) | Mg Microspheres (g) | |||
|---|---|---|---|---|---|---|---|
| MMA | DMPT | PMMA | BPO | BaSO4 | |||
| PMMA | 0 | 9.4 | 0.109 | 17.7 | 0.3 | 2 | 0 |
| PMMA–25Mg | 25.3% | 9.4 | 0.109 | 17.7 | 0.3 | 2 | 10 |
| PMMA–41Mg | 40.7% | 9.4 | 0.109 | 17.7 | 0.3 | 2 | 20 |
| PMMA–58Mg | 57.5% | 9.4 | 0.109 | 17.7 | 0.3 | 2 | 40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Q.; Han, F.; He, Z.; Shi, C.; Zhou, P.; Zhu, C.; Guo, Q.; Zhu, X.; Yang, H.; Li, B. The “Magnesium Sacrifice” Strategy Enables PMMA Bone Cement Partial Biodegradability and Osseointegration Potential. Int. J. Mol. Sci. 2018, 19, 1746. https://doi.org/10.3390/ijms19061746
Zhai Q, Han F, He Z, Shi C, Zhou P, Zhu C, Guo Q, Zhu X, Yang H, Li B. The “Magnesium Sacrifice” Strategy Enables PMMA Bone Cement Partial Biodegradability and Osseointegration Potential. International Journal of Molecular Sciences. 2018; 19(6):1746. https://doi.org/10.3390/ijms19061746
Chicago/Turabian StyleZhai, Qingpan, Fengxuan Han, Zhiwei He, Chen Shi, Pinghui Zhou, Caihong Zhu, Qianping Guo, Xuesong Zhu, Huilin Yang, and Bin Li. 2018. "The “Magnesium Sacrifice” Strategy Enables PMMA Bone Cement Partial Biodegradability and Osseointegration Potential" International Journal of Molecular Sciences 19, no. 6: 1746. https://doi.org/10.3390/ijms19061746