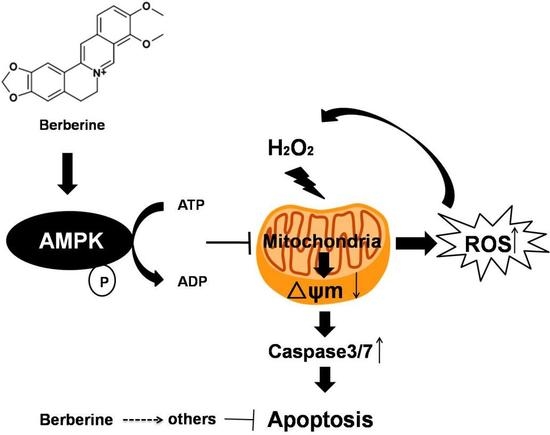
Berberine Protects Human Retinal Pigment Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Damage through Activation of AMPK
Abstract
:1. Introduction
2. Results
2.1. BBR Reduced H2O2-Induced D407 Cell Death
2.2. BBR Attenuated H2O2-Induced Apoptosis in D407 Cells
2.3. BBR Attenuated the Loss of Mitochondrial Membrane Potential and the Activation of Caspase 3/7 Induced by H2O2
2.4. BBR Attenuated the H2O2-Induced ROS Production
2.5. BBR Stimulated AMPK Phosphorylation in D407 Cells
2.6. The Protective Effect of BBR Was Mediated by AMPK Signaling
2.7. BBR Protected Primary Cultured Human RPE Cells against H2O2 Induced Injury via the AMPK Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. MTT Assay
4.4. LDH Assay
4.5. Hoechst 33342 Staining
4.6. Measurement of Cellular Oxidation (ROS)
4.7. Measurement of Mitochondrial Membrane Potential (ΔΨm)
4.8. Caspase 3/7 Activity Assay
4.9. Western Blotting
4.10. AMPK Silencing by Transfection siAMPK
4.11. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and age-related macular degeneration. Biochim. Biophys. Acta 2017, 1862, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Kauppinen, A.; Blasiak, J.; Salminen, A. Autophagy regulating kinases as potential therapeutic targets for age-related macular degeneration. Future Med. Chem. 2012, 4, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar] [PubMed]
- Chappelow, A.V.; Kaiser, P.K. Neovascular age-related macular degeneration: Potential therapies. Drugs 2008, 68, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Damico, F.M.; Gasparin, F.; Scolari, M.R.; Pedral, L.S.; Takahashi, B.S. New approaches and potential treatments for dry age-related macular degeneration. Arq. Bras. Oftalmol. 2012, 75, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weismann, D.; Hartvigsen, K.; Lauer, N.; Bennett, K.L.; Scholl, H.P.; Issa, P.C.; Cano, M.; Brandstätter, H.; Tsimikas, S.; Skerka, C. Complement factor h binds malondialdehyde epitopes and protects from oxidative stress. Nature 2011, 478, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.E.; Hariprasad, S.M.; DuPont, J.; Maguire, M.G.; Fine, S.L.; Brucker, A.J.; Maguire, A.M.; Ho, A.C. Foveolar choroidal blood flow in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1998, 39, 385–390. [Google Scholar]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.R. Rpe cell senescence: A key contributor to age-related macular degeneration. Med. Hypotheses 2012, 78, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ida, H.; Ishibashi, K.; Reiser, K.; Hjelmeland, L.M.; Handa, J.T. Ultrastructural aging of the rpe-bruch's membrane-choriocapillaris complex in the d-galactose-treated mouse. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2348–2354. [Google Scholar] [CrossRef]
- Curcio, C.A.; Saunders, P.L.; Younger, P.W.; Malek, G. Peripapillary chorioretinal atrophy: Bruch’s membrane changes and photoreceptor loss. Ophthalmology 2000, 107, 334–343. [Google Scholar] [CrossRef]
- Anderson, R.E.; Rapp, L.M.; Wiegand, R.D. Lipid peroxidation and retinal degeneration. Curr. Eye Res. 1984, 3, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Catala, A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int. J. Biochem. Cell Biol. 2006, 38, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hackett, S.F.; Mincey, A.; Lai, H.; Campochiaro, P.A. Effects of different types of oxidative stress in rpe cells. J. Cell. Physiol. 2006, 206, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Chung, J.; Yang, J.W.; Chung, S.M.; Kwag, N.H.; Yoo, J.S. Hydrogen peroxide-induced cell death in a human retinal pigment epithelial cell line, arpe-19. Korean J. Ophthalmol. 2003, 17, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, R.N.; Merwin, M.J.; Han, Z.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Yttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degeneration. Free Radic. Biol. Med. 2014, 75, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Driscoll, C.; Doonan, F.; Sanvicens, N.; Messeguer, A.; Cotter, T.G. A novel free radical scavenger rescues retinal cells in vivo. Exp. Eye Res. 2011, 93, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins c and e, beta carotene, and zinc for age-related macular degeneration and vision loss: Areds report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Chong, C.M.; Zheng, W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of erk/creb signaling. Redox Biol. 2016, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Kong, W.; Jiang, J. Learning from berberine: Treating chronic diseases through multiple targets. Sci. China Life Sci. 2015, 58, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Zhang, J.; Chen, Y.; Li, Z.; Luo, Y.; Zuo, H.; Zhao, X. Berberine sensitizes mutliple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol. Lett. 2012, 3, 1263–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.; Baggioni, A. Berberine and its role in chronic disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [PubMed]
- Lau, C.W.; Yao, X.Q.; Chen, Z.Y.; Ko, W.H.; Huang, Y. Cardiovascular actions of berberine. Cardiovasc. Drug Rev. 2001, 19, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.Y.; Chen, C.S.; Wu, S.N.; Jong, Y.J.; Lo, Y.C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012, 46, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qi, J.; Hu, R.; Chen, Y.; Kijlstra, A.; Yang, P. Effect of berberine on proinflammatory cytokine production by arpe-19 cells following stimulation with tumor necrosis factor-α. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, M.; Yu, Y.; Lan, X.; Yao, F.; Yan, X.; Chen, L.; Hatch, G.M. Berberine attenuates development of the hepatic gluconeogenesis and lipid metabolism disorder in type 2 diabetic mice and in palmitate-incubated hepg2 cells through suppression of the hnf-4alpha mir122 pathway. PLoS ONE 2016, 11, e0152097. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in h9c2 cardiomyocytes. Biochim. Biophys. Acta 2016, 1861, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Q.; Feng, Z.; Zhang, X.; Huang, B.; Chen, A.; Prestegarden, L.; Li, X.; Wang, J. Berberine induces autophagy in glioblastoma by targeting the ampk/mtor/ulk1-pathway. Oncotarget 2016, 7, 66944–66958. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Yu, J.Y.; Connell, A.R.; Yang, S.; Hookham, M.B.; McLeese, R.; Lyons, T.J. Beneficial effects of berberine on oxidized ldl-induced cytotoxicity to human retinal muller cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3369–3379. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Hsu, K.C.; Lee, J.-W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine suppresses proinflammatory responses through ampk activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Pang, T.; Gu, M.; Gao, A.-H.; Xie, C.-M.; Li, J.-Y.; Nan, F.-J.; Li, J. Berberine-stimulated glucose uptake in l6 myotubes involves both ampk and p38 mapk. Biochim. Biophys. Acta 2006, 1760, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Plafker, S.M.; O’Mealey, G.B.; Szweda, L.I. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int. Rev. Cell Mol. Biol. 2012, 298, 135–177. [Google Scholar] [PubMed]
- Shen, J.; Dong, A.; Hackett, S.; Bell, W.; Green, W.; Campochiaro, P.A. Oxidative damage in age-related macular degeneration. Histol. Histopathol. 2007, 22, 1301–1308. [Google Scholar] [PubMed]
- Biswal, M.R.; Ildefonso, C.J.; Mao, H.; Seo, S.J.; Wang, Z.; Li, H.; Le, Y.Z.; Lewin, A.S. Conditional induction of oxidative stress in rpe: A mouse model of progressive retinal degeneration. Adv. Exp. Med. Biol. 2016, 854, 31–37. [Google Scholar] [PubMed]
- Pujol-Lereis, L.M.; Schäfer, N.; Kuhn, L.B.; Rohrer, B.; Pauly, D. Interrelation between oxidative stress and complement activation in models of age-related macular degeneration. Adv. Exp. Med. Biol. 2016, 854, 87–93. [Google Scholar] [PubMed]
- Christen, W.G.; Glynn, R.J.; Sesso, H.D.; Kurth, T.; MacFadyen, J.; Bubes, V.; Buring, J.E.; Manson, J.E.; Gaziano, J.M. Vitamins e and c and medical record-confirmed age-related macular degeneration in a randomized trial of male physicians. Ophthalmology 2012, 119, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The nutritional amd treatment 2 study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long term effects of lutein, zeaxanthin and omega-3-lcpufas supplementation on optical density of macular pigment in amd patients: The lutega study. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M. Berberine protects against 6-ohda-induced neurotoxicity in pc12 cells and zebrafish through hormetic mechanisms involving pi3k/akt/bcl-2 and nrf2/ho-1 pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Su, X.; Gao, Y.; Sun, B.; Yu, Y.; Wang, X.; Zhang, F. Berberine protects mesenchymal stem cells against hypoxia-induced apoptosis in vitro. Biol. Pharm. Bull. 2009, 32, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tang, Q.; Hu, B.-R.; Xiang, J.-Z. Antioxidant properties of berberine on cultured rabbit corpus cavernosum smooth muscle cells injured by hydrogen peroxide. Acta Pharmacol. Sin. 2007, 28, 1914–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Liu, Y.; Wang, B.; Luo, Y.; Hu, N.; Chen, D.; Zhang, X.; Xiong, Y. Protective effect of berberine against oxidative stress-induced apoptosis in rat bone marrow-derived mesenchymal stem cells. Exp. Ther. Med. 2016, 12, 4041–4048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Song, J.; Wang, C.; Li, Y.; Dunaief, J.L. Berberine protects against light-induced photoreceptor degeneration in the mouse retina. Exp. Eye Res. 2016, 145, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial dysfunction in retinal diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Musat, O.; Ochinciuc, U.; Gutu, T.; Cristescu, T.; Coman, C. Pathophysiology and treatment of armd. Oftalmologia 2012, 56, 45–50. [Google Scholar] [PubMed]
- Feher, J.; Kovacs, B.; Kovacs, I.; Schveoller, M.; Papale, A.; Gabrieli, C.B. Improvement of visual functions and fundus alterations in early age-related macular degeneration treated with a combination of acetyl-l-carnitine, n-3 fatty acids, and coenzyme q10. Ophthalmologica 2005, 219, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Feher, J.; Papale, A.; Mannino, G.; Gualdi, L.; Gabrieli, C.B. Mitotropic compounds for the treatment of age-related macular degeneration. Ophthalmologica 2003, 217, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Pinto Rolo, A.; Soeiro Teodoro, J.; Furtado, E.; Caetano Oliveira, R.; Tralhão, J.; Marques Palmeira, C. Addition of berberine to preservation solution in an animal model of ex vivo liver transplant preserves mitochondrial function and bioenergetics from the damage induced by ischemia/reperfusion. Int. J. Mol. Sci. 2018, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Duarte, F.V.; Nunes, P.; Hubbard, B.P.; Teodoro, J.S.; Varela, A.T.; Jones, J.G.; Sinclair, D.A.; Palmeira, C.M.; Rolo, A.P. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing sirt1-dependent mitochondrial biogenesis. Biochim. Biophys. Acta 2012, 1822, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Dong, J. Protective effects of carnosic acid against mitochondria-mediated injury in h9c2 cardiomyocytes induced by hypoxia/reoxygenation. Exp. Ther. Med. 2017, 14, 5629–5634. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sheng, M.; Xu, R.; Yu, J.; Cui, K.; Tong, J.; Shi, L.; Ren, H.; Du, H. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J. Transl. Med. 2013, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates amp-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Domenech, E.; Maestre, C.; Esteban-Martinez, L.; Partida, D.; Pascual, R.; Fernandez-Miranda, G.; Seco, E.; Campos-Olivas, R.; Perez, M.; Megias, D.; et al. Ampk and pfkfb3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nat. Cell Biol. 2015, 17, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Wei, Y.H. Ampk-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: Implication of the cell survival in mitochondrial diseases. Biochim. Biophys. Acta 2012, 1822, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Walker, C.L. The role of lkb1 and ampk in cellular responses to stress and damage. FEBS Lett. 2011, 585, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Li, J.Y.; Gosby, A.; To, S.W.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex i: A mechanism for the action of berberine to activate amp-activated protein kinase and improve insulin action. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Bi, H.-E.; Sheng, Y.; Cheng, L.-B.; Wendu, R.-L.; Wang, C.-H.; Cao, G.-F.; Jiang, Q. Ultraviolet (uv) and hydrogen peroxide activate ceramide-er stress-ampk signaling axis to promote retinal pigment epithelium (rpe) cell apoptosis. Int. J. Mol. Sci. 2013, 14, 10355–10368. [Google Scholar] [CrossRef] [PubMed]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Gaur, U.; Chong, C.-M.; Lin, S.; Fang, J.; Zeng, Z.; Wang, H.; Zheng, W. Berberine Protects Human Retinal Pigment Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Damage through Activation of AMPK. Int. J. Mol. Sci. 2018, 19, 1736. https://doi.org/10.3390/ijms19061736
Li S, Gaur U, Chong C-M, Lin S, Fang J, Zeng Z, Wang H, Zheng W. Berberine Protects Human Retinal Pigment Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Damage through Activation of AMPK. International Journal of Molecular Sciences. 2018; 19(6):1736. https://doi.org/10.3390/ijms19061736
Chicago/Turabian StyleLi, Shuai, Uma Gaur, Cheong-Meng Chong, Shaofen Lin, Jiankang Fang, Zhiwen Zeng, Haitao Wang, and Wenhua Zheng. 2018. "Berberine Protects Human Retinal Pigment Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Damage through Activation of AMPK" International Journal of Molecular Sciences 19, no. 6: 1736. https://doi.org/10.3390/ijms19061736