NSC 95397 Suppresses Proliferation and Induces Apoptosis in Colon Cancer Cells through MKP-1 and the ERK1/2 Pathway
Abstract
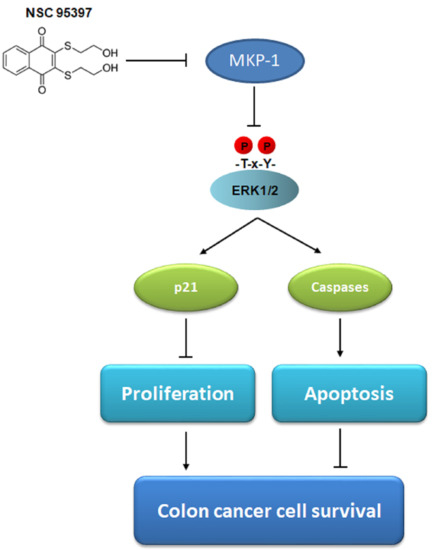
:1. Introduction
2. Results
2.1. NSC 95397 Reduces Cell Viability of Colon Cancer Cells
2.2. NSC 95397 Suppresses Soft Agar Colony Formation in Colon Cancer Cells
2.3. NSC 95397 Reduces Cell Proliferation by Inhibiting the Expression of Cell Cycle Regulatory Proteins
2.4. NSC 95397 Induces Apoptosis in Colon Cancer Cells
2.5. NSC 95397 Enhances p21 Expression and Caspase-3 Activity via ERK1/2 Activation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay
4.3. Anchorage-Independent Growth
4.4. Cell Proliferation Assay
4.5. Apoptosis Assay
4.6. Western Blot Analysis
4.7. Statistical Analysis and Replicates
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| MKP-1 | Mitogen-activated protein kinase phosphatase-1 |
| MEK | Mitogen-activated protein kinase kinase |
| ERK | Extracellular signal-regulated kinase |
| MAPK | Mitogen-activated protein kinase |
| JNK | c-Jun N-terminal kinase |
| ANOVA | Analysis of variance |
| BrdU | Bromodeoxyuridine |
| CDK | Cyclin-dependent kinase |
| Rb | Retinoblastoma protein |
| 7-AAD | 7-aminoactinomycin D |
| PARP | Poly(ADP-ribose) polymerase |
| DUSP | Dual-specificity phosphatase |
| IKK | IκB kinase |
| MKK7 | Mitogen-activated protein kinase kinase 7 |
| TBK1 | TANK-binding kinase 1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NQO1 | NADPH-quinone oxidoreductase-1 |
References
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. Map kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev. 2007, 26, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Cheng, Z.; Malbon, C.C. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003, 191, 229–237. [Google Scholar] [CrossRef]
- Vicent, S.; Garayoa, M.; Lopez-Picazo, J.M.; Lozano, M.D.; Toledo, G.; Thunnissen, F.B.; Manzano, R.G.; Montuenga, L.M. Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin. Cancer Res. 2004, 10, 3639–3649. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Franklin, C.C.; Duke, R.C.; Kraft, R.S. Human DU145 prostate cancer cells overexpressing mitogen-activated protein kinase phosphatase-1 are resistant to Fas ligand-induced mitochondrial perturbations and cellular apoptosis. Mol. Cell. Biochem. 1999, 199, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Magi-Galluzzi, C.; Mishra, R.; Fiorentino, M.; Montironi, R.; Yao, H.; Capodieci, P.; Wishnow, K.; Kaplan, I.; Stork, P.J.; Loda, M. Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab. Investig. 1997, 76, 37–51. [Google Scholar] [PubMed]
- Denkert, C.; Schmitt, W.D.; Berger, S.; Reles, A.; Pest, S.; Siegert, A.; Lichtenegger, W.; Dietel, M.; Hauptmann, S. Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int. J. Cancer 2002, 102, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Guo, J.; Kleeff, J.; Zimmermann, A.; Buchler, M.W.; Korc, M.; Friess, H. Down-regulation of the dual-specificity phosphatase MKP-1 suppresses tumorigenicity of pancreatic cancer cells. Gastroenterology 2003, 124, 1830–1845. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kwon, J.H.; Kang, S.H.; Kim, J.W.; Yang, Y.C. Increased MAPK activity and MKP-1 overexpression in human gastric adenocarcinoma. Biochem. Biophys. Res. Commun. 1998, 250, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, D.F.; Pinna, F.; Meloni, F.; Ladu, S.; Pellegrino, R.; Sini, M.; Daino, L.; Simile, M.M.; De Miglio, M.R.; Virdis, P.; et al. Dual-specificity phosphatase 1 ubiquitination in extracellular signal-regulated kinase-mediated control of growth in human hepatocellular carcinoma. Cancer Res. 2008, 68, 4192–4200. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.P.; Li, H.; Lee, M.J.; Wang, Y.P.; Kim, J.H.; Yu, G.R.; Lee, S.Y.; Leem, S.H.; Jang, K.Y.; Kim, D.G. Disruption of a regulatory loop between DUSP1 and p53 contributes to hepatocellular carcinoma development and progression. J. Hepatol. 2015, 62, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hyer, J.M.; Yu, H.; D’Silva, N.J.; Kirkwood, K.L. DUSP1 phosphatase regulates the proinflammatory milieu in head and neck squamous cell carcinoma. Cancer Res. 2014, 74, 7191–7197. [Google Scholar] [CrossRef] [PubMed]
- Moncho-Amor, V.; de Caceres, I.I.; Bandres, E.; Martinez-Poveda, B.; Orgaz, J.L.; Sanchez-Perez, I.; Zazo, S.; Rovira, A.; Albanell, J.; Jimenez, B.; et al. DUSP1/MKP1 promotes angiogenesis, invasion and metastasis in non-small-cell lung cancer. Oncogene 2011, 30, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhou, S.; Shi, L.; Liu, X.; Lin, H.; Yu, H.; Liang, X.; Tang, J.; Yu, T.; Cai, X. DUSP1 inhibits cell proliferation, metastasis and invasion and angiogenesis in gallbladder cancer. Oncotarget 2017, 8, 12133–12144. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.Y.; Wu, G.S. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007, 67, 11933–11941. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Xu, J.; Zhou, J.Y.; Liu, Y.S.; Wu, G.S. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 2006, 66, 8870–8877. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Machado-Pinilla, R.; Manguan-Garcia, C.; Belda-Iniesta, C.; Moratilla, C.; Cejas, P.; Fresno-Vara, J.A.; de Castro-Carpeno, J.; Casado, E.; Nistal, M.; et al. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene 2006, 25, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gore, A.J.; Wilson, J.L.; Korc, M. DUSP1 is a novel target for enhancing pancreatic cancer cell sensitivity to gemcitabine. PLoS ONE 2014, 9, e84982. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, H.; Song, Z.; Lin, X.; Zhang, C. Involvement of MAPK phosphatase-1 in dexamethasone-induced chemoresistance in lung cancer. J. Chemother. 2011, 23, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Shi, Y.Y.; Higgins, L.S.; Orlowski, R.Z. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 2007, 67, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Loda, M.; Capodieci, P.; Mishra, R.; Yao, H.; Corless, C.; Grigioni, W.; Wang, Y.; Magi-Galluzzi, C.; Stork, P.J. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am. J. Pathol. 1996, 149, 1553–1564. [Google Scholar] [PubMed]
- Montagut, C.; Iglesias, M.; Arumi, M.; Bellosillo, B.; Gallen, M.; Martinez-Fernandez, A.; Martinez-Aviles, L.; Canadas, I.; Dalmases, A.; Moragon, E.; et al. Mitogen-activated protein kinase phosphatase-1 (MKP-1) impairs the response to anti-epidermal growth factor receptor (EGFR) antibody cetuximab in metastatic colorectal cancer patients. Br. J. Cancer 2010, 102, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Young Kim, S.; Kim, J.; Kim, H.S.; Kim, S.M.; Kim, E.J. Mitogen-activated protein kinase phosphatase-1 inhibition and sustained extracellular signal-regulated kinase 1/2 activation in camptothecin-induced human colon cancer cell death. Cancer Biol. Ther. 2013, 14, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Nemoto, K.; Pestell, K.E.; Cooley, K.; Southwick, E.C.; Mitchell, D.A.; Furey, W.; Gussio, R.; Zaharevitz, D.W.; Joo, B.; et al. Identification of a potent and selective pharmacophore for Cdc25 dual specificity phosphatase inhibitors. Mol. Pharmacol. 2002, 61, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Peyregne, V.P.; Kar, S.; Ham, S.W.; Wang, M.; Wang, Z.; Carr, B.I. Novel hydroxyl naphthoquinones with potent Cdc25 antagonizing and growth inhibitory properties. Mol. Cancer Ther. 2005, 4, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shen, H.; Carr, B.I.; Wipf, P.; Lazo, J.S.; Pan, S.S. NAD(P)H: Quinone oxidoreductase-1-dependent and -independent cytotoxicity of potent quinone Cdc25 phosphatase inhibitors. J. Pharmacol. Exp. Ther. 2004, 309, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Vogt, A.; Oguri, T.; Lazo, J.S. Activation of the Raf-1/MEK/Erk kinase pathway by a novel Cdc25 inhibitor in human prostate cancer cells. Prostate 2004, 58, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.E.; Wickstrom, M.; Hassan, S.; Oberg, K.; Granberg, D. The cytotoxic agents NSC-95397, brefeldin a, bortezomib and sanguinarine induce apoptosis in neuroendocrine tumors in vitro. Anticancer Res. 2010, 30, 149–156. [Google Scholar] [PubMed]
- Vogt, A.; McDonald, P.R.; Tamewitz, A.; Sikorski, R.P.; Wipf, P.; Skoko, J.J., 3rd; Lazo, J.S. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol. Cancer Ther. 2008, 7, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.I.; Brummer, T.; O’Brien, P.M.; Daly, R.J. Dual-specificity phosphatases: Critical regulators with diverse cellular targets. Biochem. J. 2009, 418, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Zhang, P.; Harper, J.W.; Elledge, S.J.; Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef]
- Nigro, J.M.; Baker, S.J.; Preisinger, A.C.; Jessup, J.M.; Hostetter, R.; Cleary, K.; Bigner, S.H.; Davidson, N.; Baylin, S.; Devilee, P.; et al. Mutations in the p53 gene occur in diverse human tumour types. Nature 1989, 342, 705–708. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; Jackman, J.; Bae, I.; Myers, T.G.; Fan, S.; Mutoh, M.; Scudiero, D.A.; Monks, A.; Sausville, E.A.; Weinstein, J.N.; et al. Characterization of the p53 tumor suppressor pathway in cell lines of the national cancer institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997, 57, 4285–4300. [Google Scholar] [PubMed]
- Forbes, S.; Clements, J.; Dawson, E.; Bamford, S.; Webb, T.; Dogan, A.; Flanagan, A.; Teague, J.; Wooster, R.; Futreal, P.A.; et al. Cosmic 2005. Br. J. Cancer 2006, 94, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.R.; Rowan, A.; Smith, M.E.; Kerr, I.B.; Bodmer, W.F.; Gannon, J.V.; Lane, D.P. p53 mutations in colorectal cancer. Proc. Natl. Acad. Sci. USA 1990, 87, 7555–7559. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S. p21(WAF1) mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 2016, 76, 5189–5191. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Wu, P.; Lee, J.Y.; Li, P.R.; Hsieh, W.Y.; Ho, C.C.; Ho, C.L.; Chen, W.J.; Wang, C.C.; Yen, M.Y.; et al. Hinokitiol induces DNA damage and autophagy followed by cell cycle arrest and senescence in gefitinib-resistant lung adenocarcinoma cells. PLoS ONE 2014, 9, e104203. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Hu, J.; Yang, X.; Chen, M.; Lei, X.; Deng, L.; Yao, N.; Peng, Q.; Chen, Z.; Ye, W.; et al. Ailanthone inhibits Huh7 cancer cell growth via cell cycle arrest and apoptosis in vitro and in vivo. Sci. Rep. 2015, 5, 16185. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Enhancement of cisplatin-mediated apoptosis in ovarian cancer cells through potentiating G2/M arrest and p21 upregulation by combinatorial epigallocatechin gallate and sulforaphane. J. Oncol. 2013, 2013, 872957. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Choi, K.M.; Kim, W.; Jeon, Y.S.; Lee, Y.M.; Hong, J.T.; Yun, Y.P.; Yoo, H.S. Hinokitiol inhibits cell growth through induction of S-phase arrest and apoptosis in human colon cancer cells and suppresses tumor growth in a mouse xenograft experiment. J. Nat. Prod. 2013, 76, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Ameisen, J.C. On the origin, evolution, and nature of programmed cell death: A timeline of four billion years. Cell Death Differ. 2002, 9, 367–393. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.E.; Lovborg, H.; Rickardson, L.; Larsson, R.; Oberg, K.; Granberg, D. Identification and evaluation of potential anti-cancer drugs on human neuroendocrine tumor cell lines. Anticancer Res. 2006, 26, 4125–4129. [Google Scholar] [PubMed]
- Yang, Y.; Yang, W.S.; Yu, T.; Yi, Y.S.; Park, J.G.; Jeong, D.; Kim, J.H.; Oh, J.S.; Yoon, K.; Kim, J.H.; et al. Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases. Biochem. Pharmacol. 2014, 88, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, M.; Draetta, G.F. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 2003, 4, 671–677. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.F.; Lee, T.S.; Lin, S.Y.; Hsu, S.P.; Juan, S.H.; Hsu, Y.H.; Zhong, W.B.; Lee, W.S. Involvement of Ras/Raf-1/ERK actions in the magnolol-induced upregulation of p21 and cell-cycle arrest in colon cancer cells. Mol. Carcinog. 2007, 46, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Wu, S.; Teraishi, F.; Davis, J.J.; Jacob, D.; Fang, B. Induction of S-phase arrest and p21 overexpression by a small molecule 2[[3-(2,3-dichlorophenoxy)propyl] amino]ethanol in correlation with activation of ERK. Oncogene 2004, 23, 4984–4992. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B.; Kidd, V.J.; Mak, T.W.; Ingram, A.J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 2002, 277, 12710–12717. [Google Scholar] [CrossRef] [PubMed]
- Alexia, C.; Fallot, G.; Lasfer, M.; Schweizer-Groyer, G.; Groyer, A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem. Pharmacol. 2004, 68, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef] [PubMed]
- Bacus, S.S.; Gudkov, A.V.; Lowe, M.; Lyass, L.; Yung, Y.; Komarov, A.P.; Keyomarsi, K.; Yarden, Y.; Seger, R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 2001, 20, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Sharma, V.; Koul, N.; Sen, E. Involvement of miltefosine-mediated ERK activation in glioma cell apoptosis through Fas regulation. J. Neurochem. 2008, 107, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Phosphorylated extracellular signal-regulated kinase up-regulated p53 expression in shikonin-induced hela cell apoptosis. Chin. Med. J. 2005, 118, 671–677. [Google Scholar] [PubMed]
- Jemaa, M.; Mischitelli, M.; Fezai, M.; Almasry, M.; Faggio, C.; Lang, F. Stimulation of suicidal erythrocyte death by the CDC25 inhibitor NSC-95397. Cell. Physiol. Biochem. 2016, 40, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.J.; Nickoloff, J.A.; Chen, W.H.; Liu, H.Y.; Lo, W.C.; Chang, Y.T.; Yang, P.C.; Wu, C.W.; Williams, D.F.; Gelovani, J.G.; et al. FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming of lung cancer cells. Oncotarget 2014, 5, 9514–9529. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, N.K.; Peng, B.-Y.; Lin, C.-M.; Wang, P.D.; Wang, J.R.; Chan, C.-H.; Wei, H.-J.; Deng, W.-P. NSC 95397 Suppresses Proliferation and Induces Apoptosis in Colon Cancer Cells through MKP-1 and the ERK1/2 Pathway. Int. J. Mol. Sci. 2018, 19, 1625. https://doi.org/10.3390/ijms19061625
Dubey NK, Peng B-Y, Lin C-M, Wang PD, Wang JR, Chan C-H, Wei H-J, Deng W-P. NSC 95397 Suppresses Proliferation and Induces Apoptosis in Colon Cancer Cells through MKP-1 and the ERK1/2 Pathway. International Journal of Molecular Sciences. 2018; 19(6):1625. https://doi.org/10.3390/ijms19061625
Chicago/Turabian StyleDubey, Navneet Kumar, Bou-Yue Peng, Chien-Min Lin, Peter D. Wang, Joseph R. Wang, Chun-Hao Chan, Hong-Jian Wei, and Win-Ping Deng. 2018. "NSC 95397 Suppresses Proliferation and Induces Apoptosis in Colon Cancer Cells through MKP-1 and the ERK1/2 Pathway" International Journal of Molecular Sciences 19, no. 6: 1625. https://doi.org/10.3390/ijms19061625