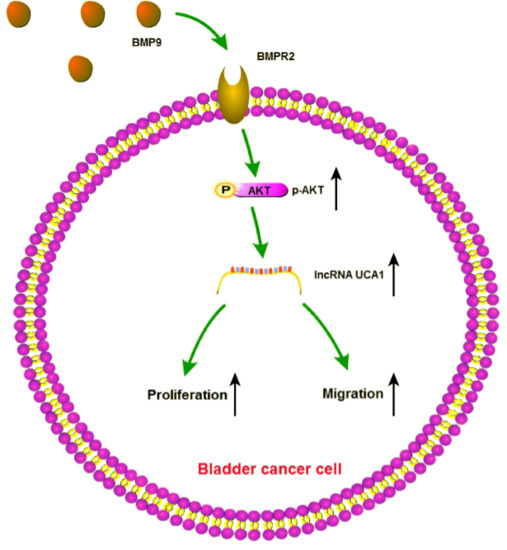
BMP9 Promotes the Proliferation and Migration of Bladder Cancer Cells through Up-Regulating lncRNA UCA1
Abstract
:1. Introduction
2. Results
2.1. The Validation of Recombinant Adenovirus BMP9 and siBMP9
2.2. BMP9 Promotes the Proliferation and Migration of Bladder Cancer Cells
2.3. BMP9 Up-Regulate the Expression of lncRNA UCA1 in Bladder Cancer Cells
2.4. BMP9 Promotes the Proliferation and Migration of Bladder Cancer BIU-87 Cells through UCA1
2.5. BMP9 Promotes the Proliferation and Migration of Bladder Cancer Cells in a UCA1 Dependent Way in Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Recombinant ADENOVIRUSES and siRNA Transfection
4.3. Western Blot Analysis
4.4. Real-Time PCR Analysis
4.5. MTT Assay
4.6. Colony Forming Test
4.7. EDU Test
4.8. Wound-Healing Test
4.9. Transwell Migration Assay
4.10. Xenograft Model
4.11. Immunohistochemistry
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Q.; Chong, T.; Yin, J.; Luo, P.; Deng, A. Molecular events are associated with resistance to vinblastine in bladder cancer. Cell. Mol. Biol. 2015, 61, 33. [Google Scholar] [PubMed]
- Soria, F.; Marra, G.; Čapoun, O.; Soukup, V.; Gontero, P. Prevention of bladder cancer incidence and recurrence: Tobacco use. Curr. Opin. Urol. 2018, 28, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Lee, D.J.; Kent, M.; Xylinas, E.; Fritsche, H.M.; Babjuk, M.; Brisuda, A.; Hansen, J.; Green, D.A.; Aziz, A.; et al. Predictors of cancer-specific mortality after disease recurrence following radical cystectomy. BJU Int. 2013, 111, E30–E36. [Google Scholar] [CrossRef] [PubMed]
- Alfred, W.J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2016, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Skinner, D.G. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J. Urol. 2006, 24, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.T.; von der Maase, H.; Sengeløv, L.; Conte, P.F.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 175, 4602. [Google Scholar]
- Zhan, Y.; Li, Y.; Guan, B.; Wang, Z.; Peng, D.; Chen, Z.; He, A.; He, S.; Gong, Y.; Li, X.; et al. Long non-coding RNA HNF1A-AS1 promotes proliferation and suppresses apoptosis of bladder cancer cells through upregulating Bcl-2. Oncotarget 2017, 8, 76656–76665. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S.P.; Goh, A. Novel endoscopic diagnosis for bladder cancer. Cancer 2015, 121, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Z.; Ke, W.; Wang, J.; Jiang, Y.; Xia, J.; Gou, L.; Liu, M.; Zhou, L.; He, T.; et al. Inhibitory effects of BMP9 on breast cancer cells by regulating their interaction with pre-adipocytes/adipocytes. Oncotarget 2017, 8, 35890. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shan, Z.; Yang, B.; Yang, D.; Men, C.; Cui, Y.; Wu, J. LncRNA HULC promotes epithelial and smooth-muscle-like differentiation of adipose-derived stem cells by upregulation of BMP9. Pharmazie 2018, 73, 49–55. [Google Scholar] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Q.; Ji, C.; Liu, X.; Li, L.; Luo, J. RUNX3 plays an important role in mediating the BMP9-induced osteogenic differentiation of mesenchymal stem cells. Int. J. Mol. Med. 2017, 40, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Y.; Zou, X.; Chen, Q.Z.; Liu, H. Follicle-Stimulating Hormone β-subunit Potentiates Bone Morphogenetic Protein 9-induced Osteogenic Differentiation in Mouse Embryonic Fibroblasts. J. Cell. Biochem. 2016, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, H.; Ren, W.; Sun, X.; Luo, J.; Tang, M.; Zhou, L.; Weng, Y.; He, T.C.; Zhang, Y. BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. J. Cancer Res. Clin. Oncol. 2011, 137, 1687. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Liu, Y.; Weng, Y.; Wang, W.; Ren, W.; Fei, C.; Chen, Y.; Zhang, Z.; Wang, T.; Wang, J.; et al. BMP9 regulates cross-talk between breast cancer cells and bone marrow-derived mesenchymal stem cells. Cell. Oncol. 2014, 37, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Weng, Y.; Zhang, M.; Li, Y.; Fan, M.; Guo, Y.; Sun, Y.; Li, W.; Shi, Q. BMP9 inhibits the growth and migration of lung adenocarcinoma A549 cells in a bone marrow stromal cell-derived microenvironment through the MAPK/ERK and NF-κB pathways. Oncol. Rep. 2016, 36, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Van, D.M.; Ten, D.P.; Inman, G.J. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009, 69, 9254–9262. [Google Scholar] [CrossRef] [PubMed]
- Garcíaálvaro, M.; Addante, A.; Roncero, C.; Fernández, M.; Fabregat, I.; Sánchez, A.; Herrera, B. BMP9-Induced Survival Effect in Liver Tumor Cells Requires p38MAPK Activation. Int. J. Mol. Sci. 2015, 16, 20431–20448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzaka, B.; Janiak, M.; Włodarski, K.H.; Radziszewski, P.; Włodarski, P.K. Expression of bone morphogenetic protein-2 and -7 in urinary bladder cancer predicts time to tumor recurrence. Arch. Med. Sci. 2015, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liao, X.; Chen, Z.; Fang, Y.; He, A.; Zhong, Y.; Gao, Q.; Xiao, H.; Li, J.; Huang, W.; et al. LncRNA MALAT1 Inhibits Apoptosis and Promotes Invasion by Antagonizing miR-125b in Bladder Cancer Cells. J. Cancer 2017, 8, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, J.; Li, H.; Yang, Y.; Yun, H.; Yang, S.; Mao, X. LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncol. Lett. 2017, 14, 5556. [Google Scholar] [CrossRef] [PubMed]
- Noon, A.P.; Catto, J.W. Noncoding RNA in bladder cancer: A specific focus upon high-risk nonmuscle invasive disease. Curr. Opin. Urol. 2014, 24, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Wu, W.; Xue, M.; Hou, H.; Zhai, W.; Chen, W. Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 2016, 382, 64. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.; Wang, Y.; Zhao, L.; Chen, W. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene 2012, 496, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Weng, Y.; Ren, W.; Zhang, Z.; Wang, T.; Wang, J.; Jiang, Y.; Chen, Y.; Zhou, L.; He, T.; et al. Biological roles of human bone morphogenetic protein 9 in the bone microenvironment of human breast cancer MDA-MB-231 cells. Am. J. Transl. Res. 2015, 7, 1660. [Google Scholar] [PubMed]
- Soloway, M.S. Editorial comment on: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur. Urol. 2008, 54, 313. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, B.W.; Burger, M.Y. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur. Urol. 2009, 56, 430. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Dinh, T.; Lee, J. The health economics of bladder cancer: An updated review of the published literature. Pharmacoeconomics 2014, 32, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Li, X.; Wu, W.; Zhang, S.; Wu, S.; Li, Z.; Chen, W. Upregulation of long non-coding RNA urothelial carcinoma associated 1 by CCAAT/enhancer binding protein α contributes to bladder cancer cell growth and reduced apoptosis. Oncol. Rep. 2014, 31, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Lee, C.T.; Montie, J.E. Bladder Cancer in 2010: How Far have We Come? Ca A Cancer J. Clin. 2010, 60, 244–272. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, C.; Yuan, T.; Liu, Y.; Song, T.; Liu, Y.; Chen, C.; Yang, M.; Tang, Z.; Shi, Q.; et al. Bone morphogenetic protein 9 regulates tumor growth of osteosarcoma cells through the Wnt/β-catenin pathway. Oncol. Rep. 2014, 31, 989. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Omrani, M.D.; Ghafouri-Fard, S. Long non-coding RNA expression in bladder cancer. Biophys. Rev. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Zhang, Z.H.; Cai, J.L.; Lv, Z.; Wang, C.; Yuan, T.; Liu, Y.; Song, T.; Liu, Y.; Chen, C.; et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 4851–4858. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Wei, S.; Zhang, S.D.; Shen, X.H.; Qiu, X.M.; Wu, H.Z.; Gong, P.H.; Lu, S.; Zhao, Z.J.; He, M.L.; et al. HBx-upregulated lncRNA UCA1 promotes cell growth and tumorigenesis by recruiting EZH2 and repressing p27Kip1/CDK2 signaling. Sci. Rep. 2016, 6, 23521. [Google Scholar] [CrossRef] [PubMed]





| Targets | Sequence | |
|---|---|---|
| β-actin | Forward | 5′-CACCACACCTTCTACAATGAGC-3′ |
| Reverse | 5′-GTGATCTCCTTCTGCATCCTGT-3′ | |
| UCA1 | Forward | 5′-CTCTCCATTGGGTTCACCATTC-3′ |
| Reverse | 5′-GCGGCAGGTCTTAAGAGATGAG-3′ | |
| H19 | Forward | 5′-ATCGGTGCCTCAGCGTTCGG-3′ |
| Reverse | 5′-CTGTCCTCGCCGTCACACCG-3′ | |
| HOTAIR | Forward | 5′-CCAGTTCTCAGGCGAGAGCC-3′ |
| Reverse | 5′-TTTATATTCAGGACATGTAA-3′ | |
| MALAT1 | Forward | 5′-ATGCGAGTTGTTCTCCGTCT-3′ |
| Reverse | 5′-TATCTGCGGTTTCCTCAAGC-3′ | |
| PVT1 | Forward | 5′-GCCCCTTCTATGGGAATCACTA-3′ |
| Reverse | 5′-GGGGCAGAGATGAAATCGTAAT-3′ | |
| siUCA1-1 | Forward | 5′-GGGCUUGGGACAUUUCACUTT-3′ |
| Reverse | 5′-AGUGAAAUGUCCCAAGCCCTT-3′ | |
| siUCA1-2 | Forward | 5′-GAGCCGAUCAGACAAACAATT-3′ |
| Reverse | 5′-UUGUUUGUCUGAUCGGCUCTT-3′ | |
| siUCA1-3 | Forward | 5′-GGGAAUACUAUUCGUAUGATT-3′ |
| Reverse | 5′-UCAUACGAAUAGUAUUCCCTT-3′ | |
| siNC | Forward | 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Reverse | 5′-ACGUGACACGUUCGGAGAATT-3′ | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, L.; Liu, M.; Xia, J.; Wan, Q.; Jiang, Y.; Sun, S.; Tang, M.; Zhou, L.; He, T.; Zhang, Y. BMP9 Promotes the Proliferation and Migration of Bladder Cancer Cells through Up-Regulating lncRNA UCA1. Int. J. Mol. Sci. 2018, 19, 1116. https://doi.org/10.3390/ijms19041116
Gou L, Liu M, Xia J, Wan Q, Jiang Y, Sun S, Tang M, Zhou L, He T, Zhang Y. BMP9 Promotes the Proliferation and Migration of Bladder Cancer Cells through Up-Regulating lncRNA UCA1. International Journal of Molecular Sciences. 2018; 19(4):1116. https://doi.org/10.3390/ijms19041116
Chicago/Turabian StyleGou, Liyao, Mengyao Liu, Jing Xia, Qun Wan, Yayun Jiang, Shilei Sun, Min Tang, Lan Zhou, Tongchuan He, and Yan Zhang. 2018. "BMP9 Promotes the Proliferation and Migration of Bladder Cancer Cells through Up-Regulating lncRNA UCA1" International Journal of Molecular Sciences 19, no. 4: 1116. https://doi.org/10.3390/ijms19041116