Zebrafish as a Model System for Investigating the Compensatory Regulation of Ionic Balance during Metabolic Acidosis
Abstract
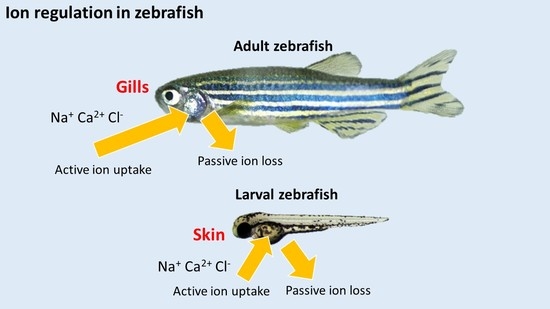
:1. Introduction
2. Physiological Responses and Compensatory Regulation of Ion Transport during Acidosis
2.1. Overview of the Effects of Acid Exposure on Freshwater Fish
2.2. Functional Regulation of Ion Transporters and Their Involvement in Ionic Compensation during Acidosis
2.2.1. Na+/H+ Exchanger (NHE)
2.2.2. Na+-Cl− Cotransporter (NCC)
2.2.3. Anion Exchanger (AE)
2.2.4. Sodium-Bicarbonate Cotransporter (NBCe)
2.2.5. Epithelial Ca2+ Channel (ECaC)
2.2.6. Effects of Acidosis on the Regulation of Other Epithelial Ion Transporters
3. Neuroendocrine Responses Following Acid Exposure
3.1. Cortisol
3.2. Endothelin
3.3. Oestrogen-Related Receptor
3.4. Catecholamines
3.5. Angiotensin II
3.6. Stanniocalcin
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwong, R.W.M.; Kumai, Y.; Perry, S.F. The physiology of fish at low pH: The zebrafish as a model system. J. Exp. Biol. 2014, 217, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Finberg, K.E.; Breton, S.; Marshansky, V.; Brown, D.; Geibel, J.P. Renal Vacuolar H+-ATPase. Physiol. Rev. 2004, 84, 1263–1314. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Devuyst, O.; Bourgeois, S.; Mohebbi, N. Regulated acid-base transport in the collecting duct. Pflügers Arch. 2009, 458, 137–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobulescu, I.A.; Moe, O.W. Na+/H+ exchangers in renal regulation of acid-base balance. Semin. Nephrol. 2006, 26, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Guh, Y.-J.; Lin, C.-H.; Hwang, P.-P. Osmoregulation in zebrafish: Ion transport mechanisms and functional regulation. EXCLI J. 2015, 14, 627–659. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hwang, P.-P. The Control of Calcium Metabolism in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2016, 17, 1783. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Kumai, Y.; Perry, S.F. Neuroendocrine control of ionic balance in zebrafish. Gen. Comp. Endocrinol. 2016, 234, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-P. Ion uptake and acid secretion in zebrafish (Danio rerio). J. Exp. Biol. 2009, 212, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Kumai, Y.; Porteus, C.S.; Tzaneva, V.; Kwong, R.W.M. An emerging role for gasotransmitters in the control of breathing and ionic regulation in fish. J. Comp. Physiol. B 2016, 186, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Guh, Y.-J.; Hwang, P.-P. Insights into molecular and cellular mechanisms of hormonal actions on fish ion regulation derived from the zebrafish model. Gen. Comp. Endocrinol. 2017, 251, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-P.; Chou, M.-Y. Zebrafish as an animal model to study ion homeostasis. Pflugers Arch. 2013, 465, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Horng, J.-L.; Kunkel, J.G.; Hwang, P.-P. Proton pump-rich cell secretes acid in skin of zebrafish larvae. Am. J. Physiol. Cell Physiol. 2005, 290, C371–C378. [Google Scholar] [CrossRef] [PubMed]
- Horng, J.-L.; Lin, L.-Y.; Huang, C.-J.; Katoh, F.; Kaneko, T.; Hwang, P.-P. Knockdown of V-ATPase subunit A (atp6v1a) impairs acid secretion and ion balance in zebrafish (Danio rerio). Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R2068–R2076. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Hirsch, S.; Gluck, S. Localization of a proton-pumping ATPase in rat kidney. J. Clin. Investig. 1988, 82, 2114–2126. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Hirscht, S.; Gluck, S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature 1988, 331, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L.; Natale, J.; Gluck, S.; Lodish, H.F.; Brown, D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc. Natl. Acad. Sci. USA 1989, 86, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-J.; Chou, M.-Y.; Kaneko, T.; Hwang, P.-P. Gene expression of Na+/H+ exchanger in zebrafish H+-ATPase-rich cells during acclimation to low-Na+ and acidic environments. Am. J. Physiol. Cell Physiol. 2007, 293, C1814–C1823. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kobayashi, S.; Nakamura, N.; Miyagi, H.; Esaki, M.; Hoshijima, K.; Hirose, S. Close Association of Carbonic Anhydrase (CA2a and CA15a), Na(+)/H(+) Exchanger (Nhe3b), and Ammonia Transporter Rhcg1 in Zebrafish Ionocytes Responsible for Na(+) Uptake. Front. Physiol. 2013, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Biemesderfer, D.; Pizzonia, J.; Abu-Alfa, A.; Exner, M.; Reilly, R.; Igarashi, P.; Aronson, P.S. NHE3: A Na+/H+ exchanger isoform of renal brush border. Am. J. Physiol. 1993, 265, F736–F742. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, M.; Loffing, J.; Lötscher, M.; Kaissling, B.; Alpern, R.J.; Moe, O.W. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995, 48, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Yan, J.-J.; Cruz, S.A.; Horng, J.-L.; Hwang, P.-P. Anion exchanger 1b, but not sodium-bicarbonate cotransporter 1b, plays a role in transport functions of zebrafish H+-ATPase-rich cells. Am. J. Physiol. Cell Physiol. 2011, 300, C295–C307. [Google Scholar] [CrossRef] [PubMed]
- Fejes-Tóth, G.; Chen, W.R.; Rusvai, E.; Moser, T.; Náray-Fejes-Tóth, A. Differential expression of AE1 in renal HCO3-secreting and -reabsorbing intercalated cells. J. Biol. Chem. 1994, 269, 26717–26721. [Google Scholar] [PubMed]
- Sussman, C.R.; Zhao, J.; Plata, C.; Lu, J.; Daly, C.; Angle, N.; DiPiero, J.; Drummond, I.A.; Liang, J.O.; Boron, W.F.; et al. Cloning, localization, and functional expression of the electrogenic Na+ bicarbonate cotransporter (NBCe1) from zebrafish. Am. J. Physiol. Cell Physiol. 2009, 297, C865–C875. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.M.; Biemesderfer, D.; Romero, M.F.; Boulpaep, E.L.; Boron, W.F. Immunolocalization of the electrogenic Na+-HCO3 cotransporter in mammalian and amphibian kidney. Am. J. Physiol. 1999, 276, F27–F38. [Google Scholar] [PubMed]
- Abuladze, N.; Song, M.; Pushkin, A.; Newman, D.; Lee, I.; Nicholas, S.; Kurtz, I. Structural organization of the human NBC1 gene: KNBC1 is transcribed from an alternative promoter in intron 3. Gene 2000, 251, 109–122. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Perry, S.F. A role for sodium-chloride cotransporters in the rapid regulation of ion uptake following acute environmental acidosis: New insights from the zebrafish model. Am. J. Physiol. Cell Physiol. 2016, 311, C931–C941. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Tseng, Y.-C.; Yan, J.-J.; Hiroi, J.; Hwang, P.-P. Role of SLC12A10.2, a Na-Cl cotransporter-like protein, in a Cl uptake mechanism in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1650–R1660. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G. The thiazide-sensitive Na+-Cl− cotransporter: Molecular biology, functional properties, and regulation by WNKs. Am. J. Physiol. Ren. Physiol. 2009, 297, F838–F848. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H.; Velázquez, H.; Wright, F.S. Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am. J. Physiol. 1987, 253, F546–F554. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, M.D.; Kaplan, M.R.; Verlander, J.W.; Lee, W.S.; Brown, D.; Poch, E.; Gullans, S.R.; Hebert, S.C. Localization of the thiazide sensitive Na-Cl cotransporter, rTSC1 in the rat kidney. Kidney Int. 1996, 50, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-C.; Liao, B.-K.; Huang, C.-J.; Lin, L.-Y.; Hwang, P.-P. Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1202–R1211. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.-K.; Deng, A.-N.; Chen, S.-C.; Chou, M.-Y.; Hwang, P.-P. Expression and water calcium dependence of calcium transporter isoforms in zebrafish gill mitochondrion-rich cells. BMC Genom. 2007, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; van der Kemp, A.W.; Hartog, A.; van de Graaf, S.F.; van Os, C.H.; Willems, P.H.; Bindels, R.J. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 1999, 274, 8375–8378. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.B.; Chen, X.Z.; Berger, U.V.; Vassilev, P.M.; Brown, E.M.; Hediger, M.A. A rat kidney-specific calcium transporter in the distal nephron. J. Biol. Chem. 2000, 275, 28186–28194. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; Hartog, A.; Stuiver, M.; Doucet, A.; Willems, P.H.; Bindels, R.J. Localization of the epithelial Ca(2+) channel in rabbit kidney and intestine. J. Am. Soc. Nephrol. 2000, 11, 1171–1178. [Google Scholar] [PubMed]
- Baxendale-Cox, L.M.; Duncan, R.L.; Liu, X.; Baldwin, K.; Els, W.J.; Helman, S.I. Steroid hormone-dependent expression of blocker-sensitive ENaCs in apical membranes of A6 epithelia. Am. J. Physiol. 1997, 273, C1650–C1656. [Google Scholar] [CrossRef] [PubMed]
- Garty, H.; Palmer, L.G. Epithelial sodium channels: Function, structure, and regulation. Physiol. Rev. 1997, 77, 359–396. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Shih, T.-H.; Liu, S.-T.; Hsu, H.-H.; Hwang, P.-P. Cortisol Regulates Acid Secretion of H(+)-ATPase-rich Ionocytes in Zebrafish (Danio rerio) Embryos. Front. Physiol. 2015, 6, 328. [Google Scholar] [CrossRef] [PubMed]
- Horng, J.-L.; Lin, L.-Y.; Hwang, P.-P. Functional regulation of H+-ATPase-rich cells in zebrafish embryos acclimated to an acidic environment. Am. J. Physiol. Cell Physiol. 2009, 296, C682–C692. [Google Scholar] [CrossRef] [PubMed]
- Fejes-Tóth, G.; Náray-Fejes-Tóth, A. Effect of acid/base balance on H-ATPase 31 kD subunit mRNA levels in collecting duct cells. Kidney Int. 1995, 48, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Verlander, J.W.; Madsen, K.M.; Cannon, J.K.; Tisher, C.C. Activation of acid-secreting intercalated cells in rabbit collecting duct with ammonium chloride loading. Am. J. Physiol. 1994, 266, F633–F645. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Perry, S.F. Ammonia excretion via Rhcg1 facilitates Na+ uptake in larval zebrafish, Danio rerio, in acidic water. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1517–R1528. [Google Scholar] [CrossRef] [PubMed]
- Laghmani, K.; Borensztein, P.; Ambühl, P.; Froissart, M.; Bichara, M.; Moe, O.W.; Alpern, R.J.; Paillard, M. Chronic metabolic acidosis enhances NHE-3 protein abundance and transport activity in the rat thick ascending limb by increasing NHE-3 mRNA. J. Clin. Investig. 1997, 99, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ambuhl, P.M.; Amemiya, M.; Danczkay, M.; Lotscher, M.; Kaissling, B.; Moe, O.W.; Preisig, P.A.; Alpern, R.J. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am. J. Physiol. 1996, 271, F917–F925. [Google Scholar] [CrossRef] [PubMed]
- Preisig, P.A.; Alpern, R.J. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J. Clin. Investig. 1988, 82, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Purkerson, J.M.; Tsuruoka, S.; Zachary Suter, D.; Nakamori, A.; Schwartz, G.J. Adaptation to metabolic acidosis and its recovery are associated with changes in anion exchanger distribution and expression in the cortical collecting duct. Kidney Int. 2010, 78, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Faroqui, S.; Sheriff, S.; Amlal, H. Metabolic acidosis has dual effects on sodium handling by rat kidney. Am. J. Physiol. Ren. Physiol. 2006, 291, F322–F331. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-J.; Wang, Y.-F.; Hu, H.-J.; Wang, J.-H.; Lee, T.-H.; Hwang, P.-P. Compensatory regulation of Na+ absorption by Na+/H+ exchanger and Na+-Cl− cotransporter in zebrafish (Danio rerio). Front. Zool. 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Kwong, R.W.M.; Perry, S.F. A role for transcription factor glial cell missing 2 in Ca2+ homeostasis in zebrafish, Danio rerio. Pflügers Arch. 2015, 467, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; Renkema, K.Y.; Hoenderop, J.G.J.; Bindels, R.J.M. Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J. Am. Soc. Nephrol. 2006, 17, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Milligan, C.L.; Wood, C.M. Disturbances in Haematology, Fluid Volume Distribution and Circulatory Function Associated with Low Environmental pH in the Rainbow Trout, Salmo Gairdneri. J. Exp. Biol. 1982, 99, 397–415. [Google Scholar]
- Wood, C.M. The physiological problems of fish in acid waters. In Acid Toxicity and Aquatic Animals; Morris, R., Taylor, E.W., Brown, D.J.A., Brown, J.A., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 125–152. [Google Scholar]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.A.; Wood, C.M. Seven things fish know about ammonia and we don’t. Respir. Physiol. Neurobiol. 2012, 184, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Perry, S.F. Mechanisms and regulation of Na+ uptake by freshwater fish. Respir. Physiol. Neurobiol. 2012, 184, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-P.; Lee, T.-H.; Lin, L.-Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef] [PubMed]
- Meneton, P. Comparative roles of the renal apical sodium transport systems in blood pressure control. J. Am. Soc. Nephrol. 2000, 11 (Suppl. S1), S135–S139. [Google Scholar] [PubMed]
- Shih, T.-H.; Horng, J.-L.; Liu, S.-T.; Hwang, P.-P.; Lin, L.-Y. Rhcg1 and NHE3b are involved in ammonium-dependent sodium uptake by zebrafish larvae acclimated to low-sodium water. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R84–R93. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Hoshijima, K.; Kobayashi, S.; Fukuda, H.; Kawakami, K.; Hirose, S. Visualization in zebrafish larvae of Na+ uptake in mitochondria-rich cells whose differentiation is dependent on foxi3a. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 292, R470–R480. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Bostanjoglo, M.; Schmitt, R.; Ellison, D.H. Sodium transport-related proteins in the mammalian distal nephron—Distribution, ontogeny and functional aspects. Anat. Embryol. 1999, 200, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Fanestil, D.D.; Vaughn, D.A.; Blakely, P. Metabolic acid-base influences on renal thiazide receptor density. Am. J. Physiol. 1997, 272, R2004–R2008. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Martin, S.W.; Fernández-Llama, P.; Masilamani, S.; Packer, R.K.; Knepper, M.A. Long-term regulation of renal Na-dependent cotransporters and ENaC: Response to altered acid-base intake. Am. J. Physiol. Ren. Physiol. 2000, 279, F459–F467. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-W.; Yang, S.-S.; Cheng, C.-J.; Tseng, M.-H.; Hsu, H.-M.; Lin, S.-H. Chronic Metabolic Acidosis Activates Renal Tubular Sodium Chloride Cotransporter through Angiotension II-dependent WNK4-SPAK Phosphorylation Pathway. Sci. Rep. 2016, 6, 18360. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Perry, S.F. The tight junction protein claudin-b regulates epithelial permeability and sodium handling in larval zebrafish, Danio rerio. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R504–R513. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, S.; Wang, Z.; Ma, L.; Soleimani, M. Regulation of the apical Cl−/HCO exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am. J. Physiol. Ren. Physiol. 2003, 284, F103–F112. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Finberg, K.E.; Stehberger, P.A.; Lifton, R.P.; Giebisch, G.H.; Aronson, P.S.; Geibel, J.P. Regulation of the expression of the Cl−/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int. 2002, 62, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Bayaa, M.; Vulesevic, B.; Esbaugh, A.; Braun, M.; Ekker, M.E.; Grosell, M.; Perry, S.F. The involvement of SLC26 anion transporters in chloride uptake in zebrafish (Danio rerio) larvae. J. Exp. Biol. 2009, 212, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Asan, E.; Jöns, T.; Kerscher, C.; Püschel, B.; Drenckhahn, D. Expression of rat kidney anion exchanger 1 in type A intercalated cells in metabolic acidosis and alkalosis. Am. J. Physiol. 1999, 277, F841–F849. [Google Scholar] [CrossRef] [PubMed]
- Boron, W.F. Acid-Base Transport by the Renal Proximal Tubule. J. Am. Soc. Nephrol. 2006, 17, 2368–2382. [Google Scholar] [CrossRef] [PubMed]
- Burnham, C.E.; Flagella, M.; Wang, Z.; Amlal, H.; Shull, G.E.; Soleimani, M. Cloning, renal distribution, and regulation of the rat Na+-HCO3− cotransporter. Am. J. Physiol. 1998, 274, F1119–F1126. [Google Scholar] [PubMed]
- Soleimani, M.; Bizal, G.L.; McKinney, T.D.; Hattabaugh, Y.J. Effect of in vitro metabolic acidosis on luminal Na+/H+ exchange and basolateral Na+:HCO3− cotransport in rabbit kidney proximal tubules. J. Clin. Investig. 1992, 90, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Inatomi, J.; Sekine, T.; Cha, S.H.; Kanai, Y.; Kunimi, M.; Tsukamoto, K.; Satoh, H.; Shimadzu, M.; Tozawa, F.; et al. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat. Genet. 1999, 23, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Dinour, D.; Chang, M.-H.; Satoh, J.; Smith, B.L.; Angle, N.; Knecht, A.; Serban, I.; Holtzman, E.J.; Romero, M.F. A Novel Missense Mutation in the Sodium Bicarbonate Cotransporter (NBCe1/SLC4A4) Causes Proximal Tubular Acidosis and Glaucoma through Ion Transport Defects. J. Biol. Chem. 2004, 279, 52238–52246. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.J.; Yuan, L.; Felmlee, M.A.; Lin, Y.-Y.; Jiang, Y.; Pei, Y.; Wang, O.; Li, M.; Xing, X.-P.; Marshall, A.; et al. A novel mutant Na+/HCO3− cotransporter NBCe1 in a case of compound-heterozygous inheritance of proximal renal tubular acidosis. J. Physiol. 2016, 594, 6267–6286. [Google Scholar] [CrossRef] [PubMed]
- Gawenis, L.R.; Bradford, E.M.; Prasad, V.; Lorenz, J.N.; Simpson, J.E.; Clarke, L.L.; Woo, A.L.; Grisham, C.; Sanford, L.P.; Doetschman, T.; et al. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J. Biol. Chem. 2007, 282, 9042–9052. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-D.; You, M.-S.; Guh, Y.-J.; Ma, M.; Jiang, Y.-J.; Hwang, P.-P. A Positive Regulatory Loop between foxi3a and foxi3b Is Essential for Specification and Differentiation of Zebrafish Epidermal Ionocytes. PLoS ONE 2007, 2, e302. [Google Scholar] [CrossRef] [PubMed]
- Van de Graaf, S.F.J.; Bindels, R.J.M.; Hoenderop, J.G.J. Physiology of epithelial Ca2+ and Mg2+ transport. Rev. Physiol. Biochem. Pharmacol. 2007, 158, 77–160. [Google Scholar] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H.; Hoenderop, J.G.J.; Bindels, R.J.M. Molecular basis of epithelial Ca2+ and Mg2+ transport: Insights from the TRP channel family. J. Physiol. 2011, 589, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Bindels, R.J.; Hartog, A.; Abrahamse, S.L.; Van Os, C.H. Effects of pH on apical calcium entry and active calcium transport in rabbit cortical collecting system. Am. J. Physiol. 1994, 266, F620–F627. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Hoenderop, J.G.J.; van Os, C.H.; Bindels, R.J.M. The epithelial calcium channel, ECaC1: Molecular details of a novel player in renal calcium handling. Nephrol. Dial. Transplant. 2001, 16, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Vanoevelen, J.; Janssens, A.; Huitema, L.F.A.; Hammond, C.L.; Metz, J.R.; Flik, G.; Voets, T.; Schulte-Merker, S. Trpv5/6 is vital for epithelial calcium uptake and bone formation. FASEB J. 2011, 25, 3197–3207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumai, Y.; Nesan, D.; Vijayan, M.M.; Perry, S.F. Cortisol regulates Na+ uptake in zebrafish, Danio rerio, larvae via the glucocorticoid receptor. Mol. Cell. Endocrinol. 2012, 364, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.A.; Lin, C.-H.; Chao, P.-L.; Hwang, P.-P. Glucocorticoid receptor, but not mineralocorticoid receptor, mediates cortisol regulation of epidermal ionocyte development and ion transport in zebrafish (Danio rerio). PLoS ONE 2013, 8, e77997. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.A.; Chao, P.-L.; Hwang, P.-P. Cortisol promotes differentiation of epidermal ionocytes through Foxi3 transcription factors in zebrafish (Danio rerio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Kwong, R.W.M.; Perry, S.F. The role of cAMP-mediated intracellular signaling in regulating Na+ uptake in zebrafish larvae. Am. J. Physiol. Integr. Comp. Physiol. 2014, 306, R51–R60. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Perry, S.F. Hydrogen sulfide promotes calcium uptake in larval zebrafish. Am. J. Physiol. Cell Physiol. 2015, 309, C60–C69. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Dehmelt, L.; Nalbant, P. Na+-dependent phosphate cotransporters: The NaPi protein families. J. Exp. Biol. 1998, 201, 3135–3142. [Google Scholar] [PubMed]
- Biber, J.; Hernando, N.; Forster, I.; Murer, H. Regulation of phosphate transport in proximal tubules. Pflügers Arch. 2009, 458, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ambühl, P.M.; Zajicek, H.K.; Wang, H.; Puttaparthi, K.; Levi, M. Regulation of renal phosphate transport by acute and chronic metabolic acidosis in the rat. Kidney Int. 1998, 53, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Nowik, M.; Picard, N.; Stange, G.; Capuano, P.; Tenenhouse, H.S.; Biber, J.; Murer, H.; Wagner, C.A. Renal phosphaturia during metabolic acidosis revisited: Molecular mechanisms for decreased renal phosphate reabsorption. Pflügers Arch. 2008, 457, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, C.; Nalbant, P.; Schölermann, B.; Hentschel, H.; Kinne, R.K.H.; Werner, A. Characterization of a type IIb sodium-phosphate cotransporter from zebrafish (Danio rerio) kidney. Am. J. Physiol. Ren. Physiol. 2003, 284, F727–F736. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, P.; Boehmer, C.; Dehmelt, L.; Wehner, F.; Werner, A. Functional characterization of a Na+-phosphate cotransporter (NaPi-II) from zebrafish and identification of related transcripts. J. Physiol. 1999, 520 Pt 1, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Beene, L.C.; Halluer, J.; Yoshinaga, M.; Hamdi, M.; Liu, Z. Pentavalent arsenate transport by zebrafish phosphate transporter NaPi-IIb1. Zebrafish 2011, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, K.J.; Murer, H. Sulphate and phosphate transport in the renal proximal tubule. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 299, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Puttaparthi, K.; Markovich, D.; Halaihel, N.; Wilson, P.; Zajicek, H.K.; Wang, H.; Biber, J.; Murer, H.; Rogers, T.; Levi, M. Metabolic acidosis regulates rat renal Na-Si cotransport activity. Am. J. Physiol. 1999, 276, C1398–C1404. [Google Scholar] [CrossRef] [PubMed]
- Markovich, D.; Romano, A.; Storelli, C.; Verri, T. Functional and structural characterization of the zebrafish Na+-sulfate cotransporter 1 (NaS1) cDNA and gene (slc13a1). Physiol. Genom. 2008, 34, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wingert, R.A.; Selleck, R.; Yu, J.; Song, H.-D.; Chen, Z.; Song, A.; Zhou, Y.; Thisse, B.; Thisse, C.; McMahon, A.P.; et al. The cdx Genes and Retinoic Acid Control the Positioning and Segmentation of the Zebrafish Pronephros. PLoS Genet. 2007, 3, e189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumz, M.L.; Lynch, I.J.; Greenlee, M.M.; Cain, B.D.; Wingo, C.S. The renal H+-K+-ATPases: Physiology, regulation, and structure. Am. J. Physiol. Renal Physiol. 2010, 298, F12–F21. [Google Scholar] [CrossRef] [PubMed]
- Cheval, L.; Morla, L.; Elalouf, J.-M.; Doucet, A. Kidney collecting duct acid-base “regulon”. Physiol. Genom. 2006, 27, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.B.; Mennitt, P.A.; Satlin, L.M. Stimulation of apical H-K-ATPase in intercalated cells of cortical collecting duct with chronic metabolic acidosis. Am. J. Physiol. 1996, 270, F539–F547. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.B.; Frindt, G.; Mennitt, P.; Satlin, L.M. Characterization and regulation of H-K-ATPase in intercalated cells of rabbit cortical collecting duct. J. Exp. Zool. 1997, 279, 443–455. [Google Scholar] [CrossRef]
- Guh, Y.-J.; Tseng, Y.-C.; Yang, C.-Y.; Hwang, P.-P. Endothelin-1 regulates H+-ATPase-dependent transepithelial H+ secretion in zebrafish. Endocrinology 2014, 155, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Bernier, N.J.; Perry, S.F. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J. Endocrinol. 2014, 220, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-Y.; Lin, C.-H.; Chao, P.-L.; Hung, J.-C.; Cruz, S.A.; Hwang, P.-P. Stanniocalcin-1 controls ion regulation functions of ion-transporting epithelium other than calcium balance. Int. J. Biol. Sci. 2015, 11, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Ward, M.A.R.; Perry, S.F. β-Adrenergic regulation of Na+ uptake by larval zebrafish Danio rerio in acidic and ion-poor environments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R1031–R1041. [Google Scholar] [CrossRef] [PubMed]
- Guh, Y.-J.; Yang, C.-Y.; Liu, S.-T.; Huang, C.-J.; Hwang, P.-P. Oestrogen-related receptor α is required for transepithelial H+ secretion in zebrafish. Proc. Biol. Sci. 2016, 283, 20152582. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Edwards, D.; Whitehead, C. Cortisol and thyroid hormone responses to acid stress in the brown trout, Salmo trutta L. J. Fish Biol. 1989, 35, 73–84. [Google Scholar] [CrossRef]
- Flik, G.; van der Velden, J.A.; Seegers, H.C.; Kolar, Z.; Wendelaar Bonga, S.E. Prolactin cell activity and sodium fluxes in tilapia (Oreochromis mossambicus) after long-term acclimation to acid water. Gen. Comp. Endocrinol. 1989, 75, 39–45. [Google Scholar] [CrossRef]
- Furukawa, F.; Watanabe, S.; Kaneko, T.; Uchida, K. Changes in gene expression levels of somatolactin in the pituitary and morphology of gill mitochondria-rich cells in Mozambique tilapia after transfer to acidic freshwater (pH 3.5). Gen. Comp. Endocrinol. 2010, 166, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, S.; Kaneko, T.; Hirano, T. Elevation of plasma somatolactin concentrations during acidosis in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1996, 199, 1043–1051. [Google Scholar]
- Briet, M.; Schiffrin, E.L. Aldosterone: Effects on the kidney and cardiovascular system. Nat. Rev. Nephrol. 2010, 6, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A. Effect of Mineralocorticoids on Acid-Base Balance. Nephron Physiol. 2014, 128, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunet, P.; Sturm, A.; Milla, S. Multiple corticosteroid receptors in fish: From old ideas to new concepts. Gen. Comp. Endocrinol. 2006, 147, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, A.K.; Butler, P.C.; White, R.B.; DeMarco, U.; Pearce, D.; Fernald, R.D. Multiple Corticosteroid Receptors in a Teleost Fish: Distinct Sequences, Expression Patterns, and Transcriptional Activities. Endocrinology 2003, 144, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Colombe, L.; Fostier, A.; Bury, N.; Pakdel, F.; Guiguen, Y. A mineralocorticoid-like receptor in the rainbow trout, Oncorhynchus mykiss: Cloning and characterization of its steroid binding domain. Steroids 2000, 65, 319–328. [Google Scholar] [CrossRef]
- Lin, C.-H.; Tsai, I.-L.; Su, C.-H.; Tseng, D.-Y.; Hwang, P.-P. Reverse Effect of Mammalian Hypocalcemic Cortisol in Fish: Cortisol Stimulates Ca2+ Uptake via Glucocorticoid Receptor-Mediated Vitamin D3 Metabolism. PLoS ONE 2011, 6, e23689. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hu, H.-J.; Hwang, P.-P. Cortisol regulates sodium homeostasis by stimulating the transcription of sodium-chloride transporter (NCC) in zebrafish (Danio rerio). Mol. Cell. Endocrinol. 2016, 422, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Perry, S.F. Cortisol regulates epithelial permeability and sodium losses in zebrafish exposed to acidic water. J. Endocrinol. 2013, 217, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E.; Inscho, E.W.; Wesson, D.; Pollock, D.M. Physiology of endothelin and the kidney. Compr. Physiol. 2011, 1, 883–919. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.; Laghmani, K.; Yanagisawa, M.; Preisig, P.A.; Alpern, R.J. An autocrine role for endothelin-1 in the regulation of proximal tubule NHE3. Kidney Int. 2004, 65, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Laghmani, K.; Preisig, P.A.; Moe, O.W.; Yanagisawa, M.; Alpern, R.J. Endothelin-1/endothelin-B receptor-mediated increases in NHE3 activity in chronic metabolic acidosis. J. Clin. Investig. 2001, 107, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.S.; Peng, Y.; Cano, A.; Yanagisawa, M.; Alpern, R.J. Endothelin(B) receptor activates NHE-3 by a Ca2+-dependent pathway in OKP cells. J. Clin. Investig. 1996, 97, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, S.; Watanabe, S.; Purkerson, J.M.; Fujimura, A.; Schwartz, G.J. Endothelin and nitric oxide mediate adaptation of the cortical collecting duct to metabolic acidosis. Am. J. Physiol. Ren. Physiol. 2006, 291, F866–F873. [Google Scholar] [CrossRef] [PubMed]
- Ranhotra, H.S. The orphan estrogen-related receptor α and metabolic regulation: New frontiers. J. Recept. Signal Transduct. 2015, 35, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.J.; Kopp, U.C.; DiBona, G.F.; Johns, E.J.; Kopp, U.C.; DiBona, G.F. Neural Control of Renal Function. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9780470650714. [Google Scholar]
- Aperia, A.; Ibarra, F.; Svensson, L.B.; Klee, C.; Greengard, P. Calcineurin mediates α-adrenergic stimulation of Na+, K+-ATPase activity in renal tubule cells. Proc. Natl. Acad. Sci. USA 1992, 89, 7394–7397. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.E.; Dubose, T.D. Adrenergic regulation of (Na+, K+)-ATPase activity in proximal tubules of spontaneously hypertensive rats. Kidney Int. 1990, 38, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gesek, F.A. α1-Adrenergic receptors activate NHE1 and NHE3 through distinct signaling pathways in epithelial cells. Am. J. Physiol. Ren. Physiol. 2001, 280, F415–F425. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Nesbitt, T.; Drezner, M.K.; Friedman, P.A.; Gesek, F.A. Proximal nephron Na+/H+ exchange is regulated by α1A- and α1B-adrenergic receptor subtypes. Mol. Pharmacol. 1997, 52, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Healy, V.; Thompson, C.; Johns, E.J. The adrenergic regulation of proximal tubular Na+/H+ exchanger 3 in the rat. Acta Physiol. 2014, 210, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Premont, R.T.; Chow, C.-W.; Blitzer, J.T.; Pitcher, J.A.; Claing, A.; Stoffel, R.H.; Barak, L.S.; Shenolikar, S.; Weinman, E.J.; et al. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 1998, 392, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Jonz, M.G.; Nurse, C.A. Epithelial mitochondria-rich cells and associated innervation in adult and developing zebrafish. J. Comp. Neurol. 2006, 497, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D.; Coffman, T.M. Recent advances involving the renin–angiotensin system. Exp. Cell Res. 2012, 318, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.P.; Xu, L.; Xu, H.; Bai, L.; Collins, J.F.; Ghishan, F.K. Effect of angiotensin-II on renal Na+/H+ exchanger-NHE3 and NHE2. Biochim. Biophys. Acta 2004, 1664, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Houillier, P.; Chambrey, R.; Achard, J.M.; Froissart, M.; Poggioli, J.; Paillard, M. Signaling pathways in the biphasic effect of angiotensin II on apical Na/H antiport activity in proximal tubule. Kidney Int. 1996, 50, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Schuster, V.L.; Kokko, J.P.; Jacobson, H.R. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J. Clin. Investig. 1984, 73, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Pech, V.; Zheng, W.; Pham, T.D.; Verlander, J.W.; Wall, S.M. Angiotensin II Activates H+-ATPase in Type A Intercalated Cells. J. Am. Soc. Nephrol. 2008, 19, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Talati, G.; Ohta, A.; Rai, T.; Sohara, E.; Naito, S.; Vandewalle, A.; Sasaki, S.; Uchida, S. Effect of angiotensin II on the WNK-OSR1/SPAK-NCC phosphorylation cascade in cultured mpkDCT cells and in vivo mouse kidney. Biochem. Biophys. Res. Commun. 2010, 393, 844–848. [Google Scholar] [CrossRef] [PubMed]
- San-Cristobal, P.; Pacheco-Alvarez, D.; Richardson, C.; Ring, A.M.; Vazquez, N.; Rafiqi, F.H.; Chari, D.; Kahle, K.T.; Leng, Q.; Bobadilla, N.A.; et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 4384–4389. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.; Mistry, A.; Hanson, L.; Mallick, R.; Hoover, R.S. Mechanisms of angiotensin II stimulation of NCC are time-dependent in mDCT15 cells. Am. J. Physiol. Ren. Physiol. 2015, 308, F720–F727. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, O.; James, K.A.; McCudden, C.R.; Segal, D.; Talebian, A.; Wagner, G.F. Stanniocalcin-1 secretion and receptor regulation in kidney cells. Am. J. Physiol. Ren. Physiol. 2008, 294, F788–F794. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Tavernini, M.M.; Yachimec, C.; Mendrick, D.L.; Alfonso, P.J.; Buergin, M.; Olsen, H.S.; Antonaccio, M.J.; Thomson, A.B.; Fedorak, R.N. Stanniocalcin: A novel protein regulating calcium and phosphate transport across mammalian intestine. Am. J. Physiol. 1998, 274, G96–G102. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.-Y.; Chou, M.-Y.; Tseng, Y.-C.; Hsiao, C.-D.; Huang, C.-J.; Kaneko, T.; Hwang, P.-P. Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 296, R549–R557. [Google Scholar] [CrossRef] [PubMed]

| Transporters | Gene Name and Cellular Localization of the Protein | Protein Identity between Zebrafish and Humans | |
|---|---|---|---|
| Zebrafish | Mammals | ||
| H+-ATPase | ATP6V1AA. HRCs (apical) [12,13] | ATP6V1A. Proximal tubular cells (apical), Type A (apical) and type B (basolateral) intercalated cells [14,15,16] | 93% |
| NHE3 | NHE3b; SLC9A3.2. HRCs (apical) [17,18] | NHE3; SLC9A3. Proximal tubular cells (apical) [19,20] | 47% |
| AE1 | AE1b; SLC4A1B. HRCs (basolateral) [21] | AE1; SLC4A1. Type A intercalated cells (basolateral) [22] | 53% |
| NBCe1 | NBCe1b; SLC4A4B. NCCCs (basolateral) [21,23] | NBCe1-A ‡; SLC4A4. Proximal tubular cells (basolateral) [24,25] | 78% |
| NCC | NCC like 2; SLC12A10.2. NCCCs (apical) [26,27] | NCC; SLC12A3. Distal convoluted tubular cells (apical) [28,29,30] | 53% |
| ECaC | ECaC; TRPV5. NaRCs (apical) [31,32] | ECaC; TRPV5. Distal convoluted tubular cells and principal cells (apical) [33,34,35] | 48% |
| ENaC | N/A * | ENaC. Principal cells (apical) [36,37] | N/A * |
| Ion Transporters | Expression Levels/Activity | |
|---|---|---|
| Zebrafish * | Mammals | |
| H+-ATPase | Chronic: ↑ H+-ATPase mRNA expression ↑ H+-ATPase activity ↑ HRCs density [12,38,39] | Acute: ↑ H+-ATPase mRNA (rabbit) [40] |
| Chronic: ↑ H+-ATPase protein abundance (rabbit) [41] | ||
| NHE3 | Chronic: ↑ nhe3b mRNA expression [38] ↑ NHE3b activity [42] | Chronic: ↑ NHE3 protein abundance (rat) [43,44] ↑ NHE activity (rat) [45] |
| AE1 | Chronic: ↑ ae1b mRNA expression [38] | Chronic ↑ AE1-expressing cells ↑ AE1 protein abundance (rabbit) [46] |
| NCC | Acute: ↔ ncc mRNA expression ↔ NCCCs density ↑ NCC activity [26] | Acute: ↔ NCC protein abundance (rat) [47] |
| Chronic: ↑ ncc mRNA expression ↑ NCCCs density [48] | Chronic: ↑ NCC protein abundance (rat) [47] | |
| ECaC | Chronic: ↑ ecac-expressing cells ↑ ECaC activity [49] | Chronic: ↓ ecac (TRPV5) mRNA expression ↓ ECaC protein abundance (mice) [50] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, L.; Kwong, R.W.M. Zebrafish as a Model System for Investigating the Compensatory Regulation of Ionic Balance during Metabolic Acidosis. Int. J. Mol. Sci. 2018, 19, 1087. https://doi.org/10.3390/ijms19041087
Lewis L, Kwong RWM. Zebrafish as a Model System for Investigating the Compensatory Regulation of Ionic Balance during Metabolic Acidosis. International Journal of Molecular Sciences. 2018; 19(4):1087. https://doi.org/10.3390/ijms19041087
Chicago/Turabian StyleLewis, Lletta, and Raymond W. M. Kwong. 2018. "Zebrafish as a Model System for Investigating the Compensatory Regulation of Ionic Balance during Metabolic Acidosis" International Journal of Molecular Sciences 19, no. 4: 1087. https://doi.org/10.3390/ijms19041087