Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs
Abstract
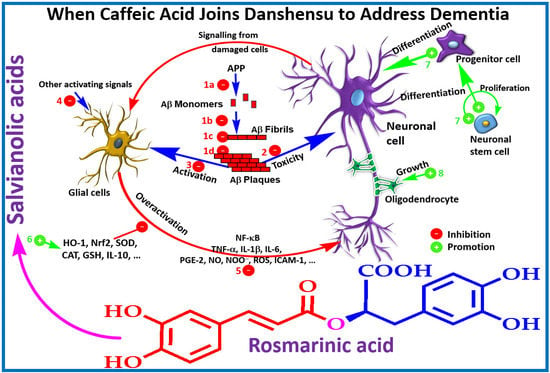
:1. Introduction
2. Natural Occurrence and Biosynthesis of Rosmarinic and Salvianolic Acids
3. Salvianolic Acids and Dementia
3.1. In Vitro Effects
3.2. Salvianolic Acids Ameliorating Dementia in Animal Models
4. Rosmarinus Acid (RA) in AD
5. Summary of Molecular Mechanisms That Attribute to the Dementia-Related Pharmacology of Salvianolic and Rosmarinic Acids
5.1. Direct Effect on Aβ Formation and Aggregation, and ROS Generation
5.2. Cholinesterase Inhibition
5.3. Tau Protein Phosphorylation and Precipitation/Aggregation
5.4. Neuronal Regeneration Mechanisms
5.5. Cell Signalling
6. Potential Drug Leads or Just Another Story of Diverse Pharmacological Effects by Polyphenols?
7. Conclusions
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report 2016, Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs now and in the Future. Available online: https://www.alz.co.uk/research/world-report-2016 (accessed on 28 January 2018).
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Available online: https://www.alz.org/facts/ (accessed on 28 January 2018).
- Hu, G.-C.; Chen, Y.-M. Post-stroke Dementia: Epidemiology, Mechanisms and Management. Int. J. Gerontol. 2017, 11, 210–214. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Association between alcohol and Alzheimer’s disease. Exp. Ther. Med. 2016, 12, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Raudino, F. Alzheimers’ Disease and Epilepsy: A Literature Review. Arch. Neurosci. 2017, 4, e39578. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Berida, T.I.; Habtemariam, S. Plants-derived neuroprotective agents: Cutting the cycle of cell death through multiple mechanisms. eCAM 2017, 2017, 3574012. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Iridoids and other monoterpenes in the Alzheimer’s brain: Recent development and future prospects. Molecules 2018, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. eCAM 2016, 2016, 2680409. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in Alzheimer’s and Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2016, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Rutin as a natural therapy for Alzheimer’s disease: Insights into its mechanisms of action. Curr. Med. Chem. 2016, 23, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Castilla, S.A.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Sureda, A.; Manayi, A.; Nabavi, S.M. Neuroprotective effects of fisetin in Alzheimer’s and Parkinson’s Diseases: From chemistry to medicine. Curr. Top. Med. Chem. 2016, 16, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Protective effects of caffeic acid and the Alzheimer’s brain: An update. Mini Rev. Med. Chem. 2017, 17, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Scarpati, M.L.; Oriente, G. Isolamento e costituzione dell’acido rosmarinico (dal rosmarinus off.). Ric. Sci. 1958, 28, 2329–2333. [Google Scholar]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; Wolters, S. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- De-Eknamkul, W.; Ellis, B.E. Tyrosine aminotransferase: The entrypoint enzyme of the tyrosine-derived pathway in rosmarinic acid biosynthesis. Phytochemistry 1987, 26, 194–1946. [Google Scholar] [CrossRef]
- Ellis, B.E.; Towers, G.H.N. Biogenesis of rosmarinic acid in Mentha. Biochem. J. 1970, 118, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, A.; Ellis, B.E. Rosmarinic acid production in Coleus cell cultures. Planta 1977, 137, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, Y.; Dong, H.; Wang, X.; Li, J.; Gao, Q. Preparation of salvianolic acid A by the degradation reaction of salvianolic acid B in subcritical water integrated with pH-zone-refining counter-current chromatography. J. Chromatogr. A. 2016, 1468, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Sun, L.; Lou, H.; Rahman, M.M. Conversion of salvianolic acid B into salvianolic acid A in tissues of Radix Salviae Miltiorrhizae using high temperature, high pressure and high humidity. Phytomedicine 2014, 21, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Song, W.-B.; Xuan, L.-J. The asymmetric total synthesis of (+)-salvianolic acid A. Tetrahedron 2016, 72, 5047–5050. [Google Scholar] [CrossRef]
- Alford, B.L.; Hügel, H.M. Total synthesis of (+)-pentamethylsalvianolic acid C. Org. Biomol. Chem. 2013, 11, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Dalla, V.; Cotelle, P. The total synthesis of salvianolic acid F. Tetrahedron 1999, 55, 6923–6930. [Google Scholar] [CrossRef]
- Wu, K.; Xie, Z.P.; Cui, D.-M.; Zhang, C. Formal total synthesis of salvianolic acid N. Org. Biomol. Chem. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.W.; Lau, K.M.; Hon, P.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel from Chinese Geriatrics Society. Recommendations on the clinical use of Compound danshen dripping pills. Chin. Med. J. 2017, 130, 972–978. [Google Scholar]
- Zhang, J.; Xie, X.; Tang, M.; Zhang, J.; Zhang, B.; Zhao, Q.; Han, Y.; Yan, W.; Peng, C.; You, Z. Salvianolic acid B promotes microglial M2-polarization and rescues neurogenesis in stress-exposed mice. Brain Behav. Immun. 2017, 66, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Hu, L.M.; Gao, X.M.; Guo, H.; Fan, G.W. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem. Res. 2010, 35, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Wang, L.; Ge, H.; Lu, X.L.; Pei, Z.; Gu, Q.; Xu, J. Salvianolic acid A, a polyphenolic derivative from Salvia miltiorrhiza bunge, as a multifunctional agent for the treatment of Alzheimer’s disease. Mol. Divers. 2013, 17, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Durairajan, S.S.K.; Chirasani, V.R.; Shetty, S.G.; Iyaswamy, A.; Malampati, S.; Song, J.; Liu, L.; Huang, J.; Senapati, S.; Li, M. Decrease in the Generation of Amyloid-β Due to Salvianolic Acid B by Modulating BACE1 Activity. Curr. Alzheimer Res. 2017, 14, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, D.; Zhang, M.H.; Zhang, W.S.; Tang, Y.X.; Shi, Z.X.; Deng, L.; Zhou, D.H.; Lu, X.Y. Salvianolic acid B inhibits Aβ generation by modulating BACE1 activity in SH-SY5Y-APPsw cells. Nutrients 2016, 8, E333. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Liu, A.H.; Wu, H.L.; Westenbroek, C.; Song, Q.L.; Yu, H.M.; Ter Horst, G.J.; Li, X.J. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents Aβ25-35-induced reduction in BPRP in PC12 cells. Biochem. Biophys. Res. Commun. 2006, 348, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.; Yu, X.; Li, Y.; Zhang, L.; He, Z.; Zhang, N.; Yang, X.; Zhao, Y.; Li, N.; et al. Salvianolic Acid B ameliorates cerebral ischemia/reperfusion injury through inhibiting TLR4/MyD88 signaling pathway. Inflammation 2016, 39, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Xu, L.; Chen, L. In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid β-induced cytotoxicity, and tanshinones act as acetylcholinesterase inhibitors. Environ. Toxicol. Pharmacol. 2011, 31, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ding, L.; Qiu, W.-F.; Wu, H.-F.; Li, R. Salvianolic acid B protects the myelin sheath around injured spinal cord axons. Neural Regen. Res. 2016, 11, 487–492. [Google Scholar] [PubMed]
- Durairajan, S.S.; Yuan, Q.; Xie, L.; Chan, W.S.; Kum, W.F.; Koo, I.; Liu, C.; Song, Y.; Huang, J.D.; Klein, W.L.; et al. Salvianolic acid B inhibits Aβ fibril formation and disaggregates preformed fibrils and protects against Aβ-induced cytotoxicty. Neurochem. Int. 2008, 52, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Chen, N.H.; Zhang, J.T. Protection of PC12 cells from hydrogen peroxide-induced cytotoxicity by salvianolic acid B, a new compound isolated from Radix Salviae miltiorrhizae. Phytomedicine 2007, 14, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhao, M.M.; Shen, P.P.; Liu, X.P.; Sun, Y.; Feng, J.C. Neuroprotective effect of salvianolic acids against cerebral ischemia/reperfusion injury. Int. J. Mol. Sci. 2016, 17, E1190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Kang, T.; Xia, Y.; Wen, Q.; Zhang, X.; Li, H.; Hu, Y.; Hao, H.; Zhao, D.; Sun, D.; et al. Effects of salvianolic acid B on survival, self-renewal and neuronal differentiation of bone marrow derived neural stem cells. Eur. J. Pharmacol. 2012, 697, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zhang, Y.; Cui, G.; Bian, Y.; Zhang, M.; Zhang, J.; Liu, Y.; Yang, X.; Isaiah, A.O.; Lin, Y.; Jiang, Y. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS ONE 2012, 7, e35636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Y.F.; Huang, Q.F.; Ge, G.L.; Cui, W. Neuroprotective effects of salvianolic acid B against oxygen-glucose deprivation/reperfusion damage in primary rat cortical neurons. Chin. Med. J. 2010, 123, 3612–3619. [Google Scholar] [PubMed]
- Guo, G.; Li, B.; Wang, Y.; Shan, A.; Shen, W.; Yuan, L.; Zhong, S. Effects of salvianolic acid B on proliferation, neurite outgrowth and differentiation of neural stem cells derived from the cerebral cortex of embryonic mice. Sci. China Life Sci. 2010, 53, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Li, G.; Zhang, S.; Gao, Y.; Jiang, W.; Fu, F.; Liu, Z. SMND-309, a novel derivate of salvianolic acid B, attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons. Basic Clin. Pharmacol. Toxicol. 2009, 104, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Musardo, S.; Marcello, E. Synaptic dysfunction in Alzheimer’s disease: From the role of amyloid β-peptide to the α-secretase ADAM10. Eur. J. Pharmacol. 2017, 817, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Z.; Sun, S.; Tan, C.C.; Yu, J.T.; Tan, L. The Role of ADAM10 in Alzheimer’s disease. J Alzheimers Dis. 2017, 58, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.R.; Zhang, H.M.; Ye, T.X.; Xiang, Z.J.; Yuan, Y.J.; Guo, Z.X.; Zhao, L.B. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 2008, 46, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, L.; Li, M.; Wu, W.; Yang, M.; Wang, J.; Guo, D.A. Salvianolic acids prevent acute doxorubicin cardiotoxicity in mice through suppression of oxidative stress. Food Chem. Toxicol. 2008, 46, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Luo, Q.; Wei, J.; Lin, R.; Lin, L.; Li, Y.; Chen, Z.; Lin, W.; Chen, Q. Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. Brain Res. 1679, 1679, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Han, B.; Geng, Y.; Wang, J.; Wang, Z.; Wang, M. Amelioration of cognitive impairments in APPswe/PS1dE9 mice is associated with metabolites alteration induced by total salvianolic acid. PLoS ONE 2017, 12, e0174763. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, C.; Zheng, Q.; Chen, S.; Li, W.; Shang, Z.; Zhang, H. Salvianolic acid A attenuates early brain injury after subarachnoid hemorrhage in rats by regulating ERK/P38/Nrf2 signaling. Am. J. Transl. Res. 2017, 9, 5643–5652. [Google Scholar] [PubMed]
- Ma, X.; Xu, W.; Zhang, Z.; Liu, N.; Yang, J.; Wang, M.; Wang, Y. Salvianolic acid B ameliorates cognitive deficits through IGF-1/Akt pathway in rats with vascular dementia. Cell. Physiol. Biochem. 2017, 43, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Cui, L.; Chen, R.; Zhang, Y.; Zhang, C.; Zhu, X.; He, T.; Shen, Z.; Dong, L.; et al. Salvianolic acids enhance cerebral angiogenesis and neurological recovery by activating JAK2/STAT3 signaling pathway after ischemic stroke in mice. J. Neurochem. 2017, 143, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhong, A.; Ma, H.; Li, D.; Hu, Y.; Xu, Y.; Zhang, J. Neuroprotective effect of salvianolic acid B against cerebral ischemic injury in rats via the CD40/NF-κB pathway associated with suppression of platelets activation and neuroinflammation. Brain Res. 2017, 1661, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Wan, Y.; Geng, S.; He, Y.; Feng, B.; Ye, Z.; Zhou, D.; Li, D.; Wei, H.; Li, H.; et al. Salvianolic Acids for Injection (SAFI) suppresses inflammatory responses in activated microglia to attenuate brain damage in focal cerebral ischemia. J. Ethnopharmacol. 2017, 198, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Wang, G.F.; Wu, G.; Wang, H.; Zhou, C.X.; Yang, H.Y.; Liu, Z.R.; Han, F.; Zhao, K. Salvianolic acid A inhibits calpain activation and eNOS uncoupling during focal cerebral ischemia in mice. Phytomedicine 2017, 25, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.S.; Wang, Y.S.; Bi, Y.L.; Guo, Z.P.; Yuan, Y.J.; Tong, S.M.; Su, R.C.; Ge, L.H.; Wang, J.; Pan, Y.L.; et al. Salvianolic acid A ameliorates the integrity of blood-spinal cord barrier via miR-101/Cul3/Nrf2/HO-1 signaling pathway. Brain Res. 2017, 1657, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Chuang, C.H.; Chern, C.M.; Liou, K.T.; Liu, D.Z.; Hou, Y.C.; Shen, Y.C. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic. Biol. Med. 2016, 99, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, L.; Shen, J.; Hao, S.; Ming, A.; Wang, X.; Su, F.; Zhang, Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res. Bull. 2015, 115, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Liu, Z.Q.; Cui, W.; Zhang, W.T.; Gong, J.P.; Wang, X.M.; Zhang, Y.; Yang, M.J. Antioxidant effect of salvianolic acid B on hippocampal CA1 neurons in mice with cerebral ischemia and reperfusion injury. Chin. J. Integr. Med. 2015, 21, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Pan, C.S.; Mao, X.W.; Liu, Y.Y.; Yan, L.; Zhou, C.M.; Fan, J.Y.; Zhang, S.Y.; Han, J.Y. Role of NADPH oxidase in total salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: Implication of AMPK/Akt/PKC. Microcirculation 2014, 21, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zou, L.; Tian, J.; Du, G.; Gao, Y. SMND-309, a novel derivative of salvianolic acid B, protects rat brains ischemia and reperfusion injury by targeting the JAK2/STAT3 pathway. Eur. J. Pharmacol. 2013, 714, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Kim, D.H.; Jeon, S.J.; Park, S.J.; Kim, J.M.; Jung, J.M.; Lee, H.E.; Bae, S.G.; Oh, H.K.; Son, K.H.; Ryu, JH. Neuroprotective effects of salvianolic acid B on an Aβ25-35 peptide-induced mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2013, 704, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, W.; Chao, X.; Zhang, L.; Qu, Y.; Huo, J.; Fei, Z. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain. Res. Bull. 2011, 84, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, S.J.; Kim, J.M.; Jeon, S.J.; Kim, D.H.; Cho, Y.W.; Son, K.H.; Lee, H.J.; Moon, J.H.; Cheong, J.H.; et al. Cognitive dysfunctions induced by a cholinergic blockade and Aβ 25-35 peptide are attenuated by salvianolic acid B. Neuropharmacology 2011, 61, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fu, F.; Li, G.; Gao, Y.; Zhang, Y.; Meng, Q.; Li, C.; Liu, F. Protections of SMND-309, a novel derivate of salvianolic acid B, on brain mitochondria contribute to injury amelioration in cerebral ischemia rats. Phytomedicine 2009, 16, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fu, F.; Li, G.; Wang, Y.; Gao, Y.; Liu, Z.; Zhang, S. SMND-309, a novel derivate of salvianolic acid B, ameliorates cerebral infarction in rats: Characterization and role. Brain Res. 2009, 1263, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Du, G.H.; Zhang, J.T. Salvianolic acid B protects brain against injuries caused by ischemia-reperfusion in rats. Acta Pharmacol. Sin. 2000, 21, 463–466. [Google Scholar] [PubMed]
- Reed, B.; Villeneuve, S.; Mack, W.; DeCarli, C.; Chui, H.C.; Jagust, W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014, 71, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, M. Cholesterol paradox: Is high total or low HDL cholesterol level a risk for Alzheimer's disease? J. Neurosci. Res. 2003, 72, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, M.E.; Landreth, G.E. Microglial interaction with β-amyloid: Implications for the pathogenesis of Alzheimer’s disease. Microsc. Res. Tech. 2001, 54, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Matas, S.; De Vera, N.; Aznar, A.O.; Marimon, J.M.; Adell, A.; Planas, A.M.; Cristofol, R.; Sanfeliu, C. In vitro and in vivo activation of astrocytes by amyloid-beta is potentiated by pro-oxidant agents. J. Alzheimers Dis. 2010, 20, 229–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Yan, Y.; Ma, L.L.; Hou, B.Y.; He, Y.Y.; Zhang, L.; Niu, Z.R.; Song, J.K.; Pang, X.C.; Yang, X.Y.; et al. Effects of the Nrf2 Protein Modulator Salvianolic Acid A Alone or Combined with Metformin on Diabetes-associated Macrovascular and Renal Injury. J. Biol. Chem. 2016, 291, 22288–22301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.Y.; Jiang, Q.; Li, K.R.; Zhao, Y.X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; An, C.; Jia, L.; Li, Y.; Chen, Q.; Zhen, F.; Wang, S.; Wang, M. Combination therapy of Salvianolic acid and fluoxetine improves the cognitive function of rats with chronic stress-induced depression. World Neurosurg. 2016, 86, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, R.; Hatayama, K.; Takahashi, T.; Hayashi, T.; Sato, Y.; Sato, D.; Ohta, K.; Nakano, H.; Seki, C.; Endo, Y.; Tokuraku, K.; Uwai, K. Structure-activity relations of rosmarinic acid derivatives for the amyloid beta aggregation inhibition and antioxidant properties. Eur. J. Med. Chem. 2017, 138, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Espargaro, A.; Ginex, T.; Vadell, M.D.; Busquets, M.A.; Estelrich, J.; Munoz-Torrero, D.; Luque, F.J.; Sabate, R. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-alzheimer drugs. J. Nat. Prod. 2017, 80, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, T.; De Filippis, D.; Esposito, G.; D’Amico, A.; Izzo, A.A. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2006, 317, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Hwang, B.R.; Lee, M.H.; Lee, S.; Cho, E.J. Perilla frutescens var. Japonica and rosmarinic acid improve amyloid-beta (25–35) induced impairment of cognition and memory function. Nutr. Res. Pract. 2016, 10, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kantar Gok, D.; Ozturk, N.; Er, H.; Aslan, M.; Demir, N.; Derin, N.; Agar, A.; Yargicoglu, P. Effects of rosmarinic acid on cognitive and biochemical alterations in ovariectomized rats treated with d-galactose. Folia Histochem. Cytobiol. 2015, 53, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Alkam, T.; Nitta, A.; Mizoguchi, H.; Itoh, A.; Nabeshima, T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by a beta(25–35). Behav. Brain Res. 2007, 180, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, A.; Aguilar Sandoval, F.; Caballero, L.; Machuca, L.; Munoz, P.; Caballero, J.; Perry, G.; Ardiles, A.; Areche, C.; Melo, F. Rosmarinic acid prevents fibrillization and diminishes vibrational modes associated to beta sheet in tau protein linked to alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2017, 32, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Wang, D.D.; Xu, Y.X.; Wang, C.; Cao, L.; Liu, Y.S.; Zhu, C.Q. Aging as a precipitating factor in chronic restraint stress-induced tau aggregation pathology, and the protective effects of rosmarinic acid. J. Alzheimers Dis. 2016, 49, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Slusarczyk, S.; Matkowski, A.; Perez-Garrido, A.; Giron-Rodriguez, F.; Ceron-Carrasco, J.P.; den-Haan, H.; Pena-Garcia, J.; Perez-Sanchez, H.; Domaradzki, K.; et al. Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia Atriplicifolia benth. and Salvia glutinosa L. Phytochemistry 2017, 133, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Demirezer, L.O.; Gurbuz, P.; Kelicen Ugur, E.P.; Bodur, M.; Ozenver, N.; Uz, A.; Guvenalp, Z. Molecular docking and ex vivo and in vitro anticholinesterase activity studies of salvia sp. And highlighted rosmarinic acid. Turk J. Med. Sci. 2015, 45, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids-interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis l. Leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, K.; Ollilainen, V.; Lackman, P.; Boije af Gennas, G.B.; Dorman, H.J.; Jarvinen, P.P.; Yli-Kauhaluoma, J.; Hiltunen, R. Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorg. Med. Chem. 2009, 17, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Haas, C. Strategies, development, and pitfalls of therapeutic options for Alzheimer’s disease. J. Alzheimers Dis. 2012, 28, 241–281. [Google Scholar] [PubMed]
- Moreira, P.I.; Zhu, X.; Nunomura, A.; Smith, M.A.; Perry, G. Therapeutic options in Alzheimer’s disease. Expert Rev. Neurother. 2006, 6, 897–910. [Google Scholar] [CrossRef] [PubMed]
- León, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; Roberts, B.R.; Streltsov, V.A.; Nuttall, S.D.; Masters, C.L. The role of Aβ in Alzheimer’s disease. In Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties; Otzen, D.E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 263–293. [Google Scholar] [CrossRef]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New pharmacological strategies for treatment of Alzheimer’s disease: Focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Fleming, J.; Tung, YC.; Lassmann, H.; Iqbal, K.; Joshi, J.G. Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol. 1990, 81, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lovell, MA.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Pereira, C.; Agostinho, P.; Moreira, P.I.; Cardoso, S.M.; Oliveira, C.R. Alzheimer’s disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 383–403. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Lentini, G. The therapeutic potential of rutin for diabetes: An update. Mini Rev. Med. Chem. 2015, 15, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Varghese, G.K. The antidiabetic therapeutic potential of dietary polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Varghese, G.K. A novel diterpene skeleton: Identification of a highly aromatic, cytotoxic and antioxidant 5-methyl-10-demethyl-abietane-type diterpene from Premna serratifolia. Phyther. Res. 2015, 29, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Investigation into the antioxidant and antidiabetic potential of Moringa stenopetala: Identification of the active principles. Nat. Prod. Commun. 2015, 10, 475–478. [Google Scholar] [PubMed]
- Habtemariam, S.; Varghese, G.K. Extractability of rutin in herbal tea preparations of Moringa stenopetala leaves. Beverages 2015, 1, 169–182. [Google Scholar] [CrossRef]
- Bose, L.V.; Varghese, G.K.; Habtemariam, S. Identification of acteoside as the active antioxidant principle of Premna serratifolia root wood tissues. Phytopharmacology 2013, 4, 228–236. [Google Scholar]
- Roselli, M.; Lentini, G.; Habtemariam, S. Phytochemical, antioxidant and anti-alpha-glucosidase activity evaluations of Bergenia cordifolia. Phyther. Res. 2012, 26, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Cowley, R.A. Antioxidant and anti-α-glucosidase ccompounds from the rhizome of Peltiphyllum peltatum (Torr.). Engl. Phytother. Res. 2012, 26, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Methyl-3-O-Methyl Gallate and Gallic Acid from the Leaves of Peltiphyllum peltatum: Isolation and Comparative Antioxidant, Prooxidant, and Cytotoxic Effects in Neuronal Cells. J. Med. Food 2011, 14, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Juan-Badaturuge, M.; Habtemariam, S.; Thomas, M.JK. Antioxidant compounds from a South Asian beverage and medicinal plant, Cassia auriculata. Food. Chem. 2011, 125, 221–225. [Google Scholar] [CrossRef]
- Juan-Badaturugea, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant principles of Tanacetum vulgare L. aerial part. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar]
- Habtemariam, S.; Dagne, E. Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med. 2010, 76, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Activity-guided isolation and identification of free Radical-scavenging components from ethanolic extract of Boneset (Leaves of Eupatorium perfoliatum). Nat. Prod. Commun. 2008, 3, 1317–1320. [Google Scholar]
- Habtemariam, S.; Jackson, C. Antioxidant and cytoprotective activity of leaves of Peltiphyllum peltatum (Torr.). Engl. Food Chem. 2007, 105, 498–503. [Google Scholar] [CrossRef]
- Habtemariam, S. Flavonoids as inhibitors or enhancers of the cytotoxicity of tumor necrosis factor-alpha in L-929 tumor cells. J. Nat. Prod. 1997, 60, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Modulation of tumour necrosis factor-α-induced cytotoxicity by polyphenols. Phyther. Res. 1997, 11, 277–280. [Google Scholar] [CrossRef]
- Habtemariam, S. Catechols and quercetin reduce MTT through iron ions: A possible artefact in cell viability assay. Phyther. Res. 1995, 9, 603–605. [Google Scholar] [CrossRef]
- Varghese, G.K.; Bose, L.V.; Habtemariam, S. Antidiabetic components of Cassia alata leaves: Identification through α-glucosidase inhibition studies. Pharm. Biol. 2013, 51, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antihyperlipidemic components of Cassia auriculata aerial parts: Identification through in vitro studies. Phytother. Res. 2013, 27, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. α-Glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat. Prod. Commun. 2011, 6, 201–203. [Google Scholar] [PubMed]
- Chen, T.; Cao, H.; Zhu, S.; Lu, Y.; Shang, Y.; Wang, M.; Tang, Y.; Zhu, L. Investigation of the binding of salvianolic acid B to human serum albumin and the effect of metal ions on the binding. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Kumar, N.; Nayak, P.G.; Nampoothiri, M.; Shenoy, R.R.; Krishnadas, N.; Rao, C.M.; Mudgal, J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J. Pharm. Pharmacol. 2013, 65, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Asha-Devi, S. Aging brain: Prevention of oxidative stress by vitamin E and exercise. Sci. World J. 2009, 9, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Murata, N.; Noda, Y.; Tahara, S.; Kaneko, T.; Kinoshita, N.; Hatsuta, H.; Murayama, S.; Barnham, K.J.; Irie, K.; Shirasawa, T.; et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid beta protein oligomerization and memory loss in mouse model of Alzheimer disease. J. Biol. Chem. 2011, 286, 44557–44568. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, P.; Martín-Aragón, S.; Benedí, J.; Susín, C.; Felici, E.; Gil, P.; Ribera, J.M.; Villar, A.M. Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from mild cognitive impairment. Free Rad. Res. 2008, 42, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Shenvi, S.; Hagen, T.M.; Liu, R.M. Glutathione metabolism during aging and in Alzheimer disease. Ann. N. Y. Acad. Sci. 2014, 1019, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Garwood, C.J.; Ratcliffe, L.E.; Simpson, J.E.; Heath, P.R.; Ince, P.G.; Wharton, S.B. Astrocytes in Alzheimer’s disease and other age-associated dementias: A supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017, 43, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, L.; Heinen, Y.; Van Dam, A.M.; Lucassen, P.J.; Korosi, A. Microglial priming and Alzheimer’s disease: A possible role for (early) immune challenges and epigenetics? Front. Hum. Neurosci. 2016, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Tago, H.; Mcgeer, E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef]
- Schwab, C.; McGeer, P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimers Dis. 2008, 13, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, R.; Gao, K.; Wei, Z.; Yin, M.Y.; Lau, L.T.; Chui, D.; Yu, A.C. Astrocytes: Implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Shamim, D.; Laskowski, M. Inhibition of inflammation mediated through the tumor Necrosis factor-α biochemical pathway can lead to favorable outcomes in Alzheimer disease. J. Cent. Nerv. Syst. Dis. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting tumor necrosis factor-α for Alzheimer’s disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Han, Y.W.; Han, X.H.; Zhang, K.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Ting, C.; Zhen, Z.; Hong, W. Upstream regulators and downstream effectors of NF-κB in Alzheimer’s disease. J. Neurol. Sci. 2016, 366, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef] [PubMed]
- Prokop, S.; Miller, K.R.; Heppner, F.L. Microglia actions in Alzheimer’s disease. Acta Neuropathol. 2013, 126, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L., Jr.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. “Alzheimer’s disease”. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Coyle, J.; Price, D.; de Long, M. Alzheimer’s disease: A disorder of cortical cholinergic innervations. Science 1983, 219, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterases: New roles in brain function and in Alzheimer’s disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Mi, K.; Johnson, G.V. The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 5562–5566. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Martinez, L.; Farías, G.A.; Maccioni, R.B. Tau oligomers as potential targets for Alzheimer’s diagnosis and novel drugs. Front. Neurol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Riemens, R.J.M.; Soares, E.S.; Esteller, M.; Delgado-Morales, R. Stem Cell Technology for (Epi)genetic Brain Disorders. Adv. Exp. Med. Biol. 2017, 978, 443–475. [Google Scholar] [PubMed]
- Tong, G.; Izquierdo, P.; Raashid, R.A. Human induced pluripotent stem cells and the modelling of Alzheimer’s disease: The human brain outside the dish. Open Neurol. J. 2017, 11, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Bendriem, R.M.; Wu, W.W.; Shen, R.F. 3D brain Organoids derived from pluripotent stem cells: Promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.; Ammit, A.J. Targeting p38 mapk pathway for the treatment of Alzheimer’s disease. Neuropharmacology 2010, 58, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Kim, N.-J. Recent Advances in the Inhibition of p38 MAPK as a Potential Strategy for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, S.A.; Eales, K.L. The Role of p38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease. J. Signal Transduct. 2012, 2012, 649079. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015, 10, 23–42. [Google Scholar]
- Tang, Y.L.; Zhang, Y.Q. Molecular mechanisms of NMDA receptor-MAPK-CREB pathway underlying the involvement of the anterior cingulate cortex in pain-related aversion. Sheng Li Xue Bao 2017, 69, 637–646. [Google Scholar] [PubMed]
- Jha, S.K.; Jha, N.K.; Kar, R. Ambasta, R.K.; Kumar, P. p38 MAPK and PI3K/AKT signalling cascades in Parkinson’s disease. Int. J. Mol. Cell. Med. 2015, 4, 67–86. [Google Scholar] [PubMed]
- Álvarez, Á.L.; Habtemariam, S.; Juan-Badaturuge, M.; Jackson, C.; Parra, F. In vitro anti HSV-1 and HSV-2 activity of Tanacetum vulgare extracts and isolated compounds: An approach to their mechanisms of action. Phytother. Res. 2011, 25, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xie, X.; Li, D.; Yu, Z.; Tong, L.; Zhao, Y. Simultaneous determination and tissue distribution studies of four phenolic acids in rat tissue by UFLC-MS/MS after intravenous administration of salvianolic acid for injection. Biomed. Chromatogr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, Y.; Tang, L.; Wang, J.; Guo, D.; Wang, M.; Zhang, X. Evaluation of brain targeting in rats of salvianolic acid B nasal delivery by the microdialysis technique. Xenobiotica 2017. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.Q.; Aa, N.; Geng, J.L.; Huang, J.Q.; Sun, R.B.; Ge, C.; Yang, Z.J.; Wang, L.S.; Aa, J.Y.; Wang, G.J. Pharmacokinetic and metabolomic analyses of the neuroprotective effects of salvianolic acid A in a rat ischemic stroke model. Acta Pharmacol. Sin. 2017, 38, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J. Another Alzheimer’s Drug Flops in Pivotal Clinical Trial. Science News. Available online: http://www.sciencemag.org/news/2017/02/another-alzheimers-drug-flops-pivotal-clinical-trial (accessed on 31 January 2018).





| Compound | Model | Outcome | Reference |
|---|---|---|---|
| SA-B | LPS-stimulated primary microglial cells from mice | 40 µM—Inhibit microglial activation; enhance neural precursor cell proliferation, differentiation, and survival; inhibit NF-κB activation along with ROS, NO, iNOS and cytokine (IL-1β, TNF-α and IL-6) production. | [29,30] |
| SA-A | SH-SY5Y cells treated with Aβ; Aβ aggregation assay | Cytoprotective; inhibit Aβ self-aggregation; disaggregates pre-formed fibrils; reduce metal-induced aggregation through chelating metal ions; reduce iROS. | [31] |
| SA-B | N2a-mouse and H4-human neuroglioma cell lines expressing SwedAPP cells | Decrease extracellular Aβ, soluble APPβ, and intracellular C-terminal fragment β from APP; no effect on α-secretase and γ-secretase activity and the levels of FL-APP; protein-docking model show interactions with the BACE1 catalytic centre. | [32] |
| SA-B | SH-SY5Y-APPsw cells | (25, 50, or 100 µM)—Reduce Aβ1–40 and Aβ1–42 level in culture media; decrease the protein expressions of BACE1 and sAPPβ; ADAM10 and sAPPα; inhibit GSK3β activity; attenuate oxidative stress (enhance SOD and GPx activities). | [33] |
| SA-B | Aβ25–35-treated PC12 cells | 20 µM—Reverse the reduced expression level of BPRP; increase cell viability; reduce ROS and iCa2+. | [34] |
| SA-B | oxygen-glucose deprivation and reoxygenation (OGD/R) model in PC12 cells and primary cortical neurons | Ameliorate NeuN protein release; inhibit the TLR4/MyD88/TRAF6 signaling pathway; inhibit NF-κB transcriptional activity and pro-inflammatory cytokine (IL-1β, IL-6, and TNF-α). | [35] |
| SA-B | Primary culture of oligodendrocyte precursor cells from rats | 20 μg/mL—promote differentiation. | [36] |
| SA-B | Aβ25–35-treated PC12 cells; enzyme assay | 200 μg/mL—Revise cytotoxicity; Ca2+-intake and LDH release; inhibit AChE. | [37] |
| SA-B | Aβ1–40 fibril formation and destabilization; Aβ1–40-treated SH-SY5Y cells | Inhibit fibril aggregation (IC50: 1.54–5.37 µM); destabilize preformed Aβ fibril (IC50: 5.00–5.19 µM); inhibit cytotoxicity (above one μM). | [38] |
| SA-B | H2O2-treated PC12 cells | (0.1–10 µM)—Pre-treatment—Improve cell survival and activities of SOD, CAT and GPx; suppress MDA, LDH, iCa2+, caspase-3 activity and apoptosis. | [39] |
| Salvianolic acid of commercial source—undescribed | Primary astrocytes from rats—OGD-induced mitochondria damage | Cytoprotective and reverse ΔΨ(m) reduction | [40] |
| SA-B | Bone marrow derived neural stem cells | Induce BDNF production; protect cells from H2O2 toxicity; promote self-renewal and neuronal differentiation. | [41] |
| SA-B | Neural stem/progenitor cells | 5–50 µM—Promote proliferation; up-regulate the expression of nestin; maintain self-renewal; effect mediated via PI3K/Akt pathway. | [42] |
| SA-B | OGD/RP-induced damage in primary rat cortical neurons | Enhance cell viability and the activities of Mn-SOD, CAT and GPx; elevate ΔΨ(m) (p < 0.01) and depress the release of cytochrome c; reverse neuronal morphological injury. | [43] |
| SA-B | NSCs from mice | 20 and 40 µg/mL—Increase the number of NSCs and their derivative neurospheres; increase G2/S-phase cell population; promote neurite outgrowth, proliferation and differentiation of NSCs. | [44] |
| SMND-309 (see Figure 4) | Cultured rat cortical neuron under OGD | 3–100 µM—Increase cell survival rate, mitochondrial antioxidant enzyme activities, respiratory enzymes activities, respiratory control ratio and ATP content; decrease mitochondrial MDA content, LDH release, iCa2+ level and caspase-3 activity. | [45] |
| Compound | Model | Outcome | Reference |
|---|---|---|---|
| SA-B | Ischemia/reperfusion injury model in mice—20, 40 or 60 mg/kg during reperfusion | Neuroprotective—decrease ROS level; suppress the expression of GFAP, Iba1, IL-1β, IL-6, TNF-α and cleaved-caspase 3; inhibit astrocytes and microglia overactivation. | [50] |
| Total salvianolic acid (commercial source) | APPswe/PS1dE mice model—30 and 60 mg/kg for 14 weeks | Improve learning and memory; decrease the LDL-C and cholesterol (higher dose) levels; decrease Aβ42 and Aβ40 levels in the hippocampus; increase glucose-6-phosphate, sucrose-6-phosphate, sorbitol, ascorbate (higher dose); reduce galactose and cholecalciferol in the hippocampus. | [51] |
| SA-A | Subarachnoid hemorrhage model in rats—10 or 50 mg/kg, i.p. | Reduce the elevated levels of ROS and MDA; increase GPx activity and GSH and BDNF in the cortex; decrease the release of inflammation cytokines (TNF-α, IL-1β, IL-6, and IL-8); reverse the decreased expression of Nrf2 and its downstream targets (HO-1 and NQO-1); No effect on phosphorylation of JNK but reversed the increased the phosphorylation of p38 MAPK and the decreased the phosphorylation of ERK. | [52] |
| SA-B | Vascular dementia model (permanent bilateral common carotid artery occlusion) in rats—20 mg/kg, p.o. for 6 weeks | Reverse the reduced hippocampal IGF-1 levels; increase phosphorylated-Akt level (Akt level not altered); inhibit apoptosis of hippocampal neurons in CA1 region. | [53] |
| Total Salvianolic acid | Angiogenesis and long-term neurological recovery after ischemic stroke—permanent distal middle cerebral artery occlusion—2 weeks treatment model. | Enhanced post-stroke angiogenesis, pericytes and astrocytic end feet covered ratio in the peri-infarct area; effects dependent on activation of JAK2/STAT3 signaling pathway. | [54] |
| SA-B | Chronic mild stress model in mice—20 mg/kg, i.p. for 3 weeks | Alter M1 microglial polarization toward M2 activation in the hippocampus and cortex; alleviate neuronal deficits in hippocampus; suppress pro-inflammatory markers (IL-1β, IFN-γ, IL-6 and iNOS,); reverse the decrease in IL-4 in both the hippocampus and the cortex; decrease the ratio of (IL-6+-Iba1+)/Iba1+ cells, and increased the ratio of (Arg-1+-Iba1+)/Iba1+ cells in hippocampus. | [29] |
| SA-B | Ischemia/reperfusion (I/R—transient middle cerebral artery occlusion) injury model in rats—3, 6 or 12 mg/kg, i.p. | Decrease I/R-induced neurological deficits, plasma-soluble P-selectin and soluble CD40 ligand, neuronal and DNA damage in the hippocampal CA1 region and neural cell loss in the ischemic core; inhibit mRNA and protein overexpression in the penumbra cortex, including ICAM-1, IL-1β, IL-6, IL-8, and MCP-1; reduce CD40 expression and NF-κB activation | [55] |
| Salvianolic Acids for Injections—crude mixture predominantly SA-B. (commercial source) | Ischemia/reperfusion or focal cerebral ischemia model—23 or 46 mg/kg, i.p. for 4 days—pretreatment | Decrease neuroinflammation and infarction volume; inhibit microglia activation along with TLR4/NF-κB-dependent release of cytokines (IL-1β and IL-6). | [56] |
| SA-A | Focal cerebral ischemia (transit middle cerebral artery occlusion mice) model in mice—1 or 5 mg/kg, i.p. | Ameliorate neuronal damage, neurological deficit and volume of infarction; inhibit eNOS uncoupling and calpain proteolytic activity; suppress peroxynitrite generation; increase AKT, FKHR and ERK phosphorylation. | [57] |
| SA-A | Blood-spinal cord barrier (BSCB) in spinal cord injury model in rats—2.5, 5 or 10 mg/kg, i.p. | Neuroprotective effect via the expression of microRNA-101 (miR-101) under hypoxia; increase Nrf2 and HO-1 expression; improve the recovery of neurological function. | [58] |
| SA-A | Ischemic brain injury model in mice—50 and 100 μg/kg, i.v. | Neuroprotective and preserves the BBB; reduce oxidative stress and apoptosis; promote endogenous neurogenesis; reverse the expression levels of DCX and Bcl-2; suppress NF-κB signaling and inflammation/nitrosative stress; promote neurogenesis-related protein expression by modulating GSK3β/Cdk5 activity; enhance the expression levels of β-catenin/DCX and Bcl-2 for neuroprotection. | [59] |
| Commercially available salvianolic acid—undescribed | Cerebral infarction of I/R (MCAO model)—10 mg/kg injection | Neuroprotection via antioxidant mechanism (increased SOD and suppressed MDA levels); upregulate mtCx43 through PI3K/AKT pathway. | [40] |
| SA-B | MCAO model | Prevent gross cerebral I/R injury. | [35] |
| SA-B | Rat model of contusion by heavy impact to induce spinal cord injury—20 mg/kg, i.p. for 8 weeks | Increase myelin sheath and the number of regenerating axons; restore neurological function; decrease caspase-3 expression in the spinal cord. | [36] |
| SA-B | MCAO model—25 mg/kg administrated twice | Cerebral-protective effect—reduce infarct volume, lower brain oedema; increase neurological scores; decrease TNF-α and IL-1β levels in brain tissue; upregulate the expression of SIRT1 and Bcl-2; downregulate the expression of Ac-FOXO1 and Bax; effects abolished by SIRT1 inhibitor (EX527). | [60] |
| SA-B | Mouse model of cerebral ischemia and reperfusion injury (bilateral carotid artery occlusion)—22.5 mg/kg | Decrease MDA content and NOS activity of the pallium; increase SOD activity and the total antioxidant capability of the pallium. | [61] |
| Total salvianolic acids (commercial source) | MCAO model in rats—1.67 mg/kg, i.p. administrated before reperfusion | Attenuate I/R-induced microcirculatory disturbance and neuron damage; activate AMPK, inhibit NADPH oxidase subunits membrane translocation, suppress Akt phosphorylation and PKC translocation. | [62] |
| SMND-309 (see Figure 4) | MCAO model in the rats | Decrease infract volume; improve neurological function and neuronal survival; promote angiogenesis by increasing the levels of erythropoietin (EPO), erythropoietin receptor (EPOR), phosphorylated JAK2 and STAT3, VEGF and VEGF receptor 2 (Flk-1) in the brain. | [63] |
| SA-A | Transgenic Caenorhabditis elegans | Inhibit Aβ-induced paralysis. | [31] |
| SA-B | Aβ25–35 injected intracerebroventricularly in mouse—10 mg/kg, p.o. for 7 days | Reverse memory impairment in the passive avoidance task; reduce microglia and astrocytes activation; reduce iNOS and COX-2 expression and TBRS level; restore ChAT and BDNF protein levels. | [64] |
| SA-B | Traumatic brain injury in mice in cortical impact model—25 mg/kg, i.v. | Reduce brain oedema, lesion volume and motor functional deficits; improve spatial learning and memory; inhibit the neutrophil infiltration and microglial activation; suppress the expression of pro-inflammatory cytokines (TNF-α and IL-1β) and enhance the expression of anti-inflammatory cytokines (IL-10 and TGF-β1) in brain tissues. | [65] |
| SA-B | Transient global ischemia in rats via irreversibly vertebral arteries occlusion—50 mg/kg, i.p. for 4 weeks | Protect learning and memory functions. | [42] |
| SA-B | Drug-induced amnesic models induced by scopolamine, diazepam, muscimol, or Aβ25–35—10 mg/kg, p.o. | Reverse cognitive impairments induced by scopolamine or Aβ25–35; Effect via the GABAergic neurotransmitter system. | [66] |
| SMND-309 | MCAO model in rats—2.5, 5 or 10 mg/kg i.v. 3 and 12 h after occlusion | Decrease neurological deficit scores, reduce the number of dead hippocampal neuronal cells, mitochondria swelling and ROS production; mmp level and mitochondrial respiratory chain complex activities; at 25.0 mg/kg—neuroprotective effect still present 7 days after ischemia. | [67,68] |
| SA-B | Cerebral ischemia-reperfusion model in rats via carotid artery occlusion—10 mg/kg i.v. | Inhibit the decrease in SOD, GSH, and ATP levels and the increase in MDA and lactic acid levels. | [69] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, 458. https://doi.org/10.3390/ijms19020458
Habtemariam S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. International Journal of Molecular Sciences. 2018; 19(2):458. https://doi.org/10.3390/ijms19020458
Chicago/Turabian StyleHabtemariam, Solomon. 2018. "Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs" International Journal of Molecular Sciences 19, no. 2: 458. https://doi.org/10.3390/ijms19020458