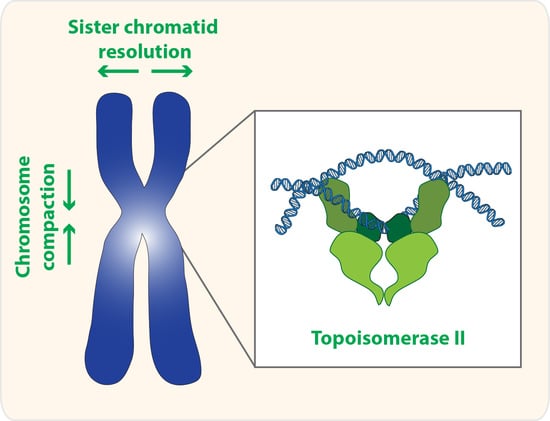
A Topology-Centric View on Mitotic Chromosome Architecture
Abstract
:1. Introduction
2. Topoisomerase II and Sister Chromatid Resolution
3. Topoisomerase II and Chromosome Compaction
4. Topoisomerase II and Biophysical Properties of Chromosomes
5. Regulation of Topoisomerase II Activity: Guidance by the Structural Maintenance of Chromosomes (SMC) Complexes
5.1. The Directionality Problem
5.2. Bacterial SMCs
5.3. SMC5/6
5.4. Cohesin—The Resolution Blocker
5.5. Condensin—The Guiding Complex
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SMC | Structural maintenance of chromosomes |
| UBF | Ultra-fine bridges |
| BLM | Bloom syndrome protein |
| PICH | Polo-like kinase 1-interacting checkpoint helicase |
References
- Goto, T.; Wang, J.C. Yeast DNA topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J. Biol. Chem. 1982, 257, 5866–5872. [Google Scholar] [PubMed]
- Hsieh, T.; Brutlag, D. ATP-dependent DNA topoisomerase from D. melanogaster reversibly catenates duplex DNA rings. Cell 1980, 21, 115–125. [Google Scholar] [CrossRef]
- Bauer, D.L.V.; Marie, R.; Rasmussen, K.H.; Kristensen, A.; Mir, K.U. DNA catenation maintains structure of human metaphase chromosomes. Nucleic Acids Res. 2012, 40, 11428–11434. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Ohkura, H.; Adachi, Y.; Morino, K.; Shiozaki, K.; Yanagida, M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell 1987, 50, 917–925. [Google Scholar] [CrossRef]
- Clarke, D.J.; Johnson, R.T.; Downes, C.S. Topoisomerase II inhibition prevents anaphase chromatid segregation in mammalian cells independently of the generation of DNA strand breaks. J. Cell Sci. 1993, 105, 563–569. [Google Scholar] [PubMed]
- Oliveira, R.A.; Hamilton, R.S.; Pauli, A.; Davis, I.; Nasmyth, K. Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei. Nat. Cell Biol. 2010, 12, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.A.; Queiroz-Machado, J.; Carmo, A.M.; Moutinho-Pereira, S.; Maiato, H.; Sunkel, C.E. Dual Role of Topoisomerase II in Centromere Resolution and Aurora B Activity. PLoS Biol. 2008, 6, e207. [Google Scholar] [CrossRef] [PubMed]
- Charbin, A.; Bouchoux, C.; Uhlmann, F. Condensin aids sister chromatid decatenation by topoisomerase II. Nucleic Acids Res. 2014, 42, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Downes, C.S.; Clarke, D.J.; Mullinger, A.M.; Gimenez-Abian, J.F.; Creighton, A.M.; Johnson, R.T. A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature 1994, 372, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Abián, J.F.; Clarke, D.J.; Devlin, J.; Giménez-Abián, M.I.; De la Torre, C.; Johnson, R.T.; Mullinger, A.M.; Downes, C.S. Premitotic chromosome individualization in mammalian cells depends on topoisomerase II activity. Chromosoma 2000, 109, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; Giménez-Abián, J.F. Checkpoints controlling mitosis. BioEssays 2000, 22, 351–363. [Google Scholar] [CrossRef]
- Furniss, K.L.; Tsai, H.-J.; Byl, J.A.W.; Lane, A.B.; Vas, A.C.; Hsu, W.-S.; Osheroff, N.; Clarke, D.J. Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation. PLoS Genet. 2013, 9, e1003832. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.J.; Karaca, G.F.; Zhou, Y.; Simpson, D.A.; Cordeiro-Stone, M.; Kaufmann, W.K. Topoisomerase IIα maintains genomic stability through decatenation G(2) checkpoint signaling. Oncogene 2010, 29, 4787–4799. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, K.; Hossain, M.J.; Roberti, M.J.; Ellenberg, J.; Hirota, T. Sister chromatid resolution is an intrinsic part of chromosome organization in prophase. Nat. Cell Biol. 2016, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zickler, D.; Prentiss, M.; Chang, F.S.; Witz, G.; Maeshima, K.; Kleckner, N. Chromosomes Progress to Metaphase in Multiple Discrete Steps via Global Compaction/Expansion Cycles. Cell 2015, 161, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Koshland, D.; Hartwell, L.H. The structure of sister minichromosome DNA before anaphase in Saccharomyces cerevisiae. Science 1987, 238, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.; Sen, N.; Martinez, V.L.; De Carandini, M.E.M.; Schvartzman, J.B.; Diffley, J.F.X.; Aragon, L. Positive Supercoiling of Mitotic DNA Drives Decatenation by Topoisomerase II in Eukaryotes. Science 2011, 331, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Leonard, J.; Torres, R.; Garcia-Luis, J.; Palou-Marin, G.; Aragón, L. Physical Proximity of Sister Chromatids Promotes Top2-Dependent Intertwining. Mol. Cell 2016, 64, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Piskadlo, E.; Tavares, A.; Oliveira, R.A. Metaphase chromosome structure is dynamically maintained by condensin I-directed DNA (de)catenation. Elife 2017, 6, e26120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nielsen, C.F.; Yao, Q.; Hickson, I.D. The origins and processing of ultra fine anaphase DNA bridges. Curr. Opin. Genet. Dev. 2014, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; North, P.S.; Hickson, I.D. BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J. 2007, 26, 3397–3409. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Körner, R.; Hofmann, K.; Nigg, E.A. PICH, a Centromere-Associated SNF2 Family ATPase, Is Regulated by Plk1 and Required for the Spindle Checkpoint. Cell 2007, 128, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Palmai-Pallag, T.; Ying, S.; Hickson, I.D. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat. Cell Biol. 2009, 11, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Naim, V.; Rosselli, F. The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat. Cell Biol. 2009, 11, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F. Portrait of replication stress viewed from telomeres. Cancer Sci. 2013, 104, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Barefield, C.; Karlseder, J. The BLM helicase contributes to telomere maintenance through processing of late-replicating intermediate structures. Nucleic Acids Res. 2012, 40, 7358–7367. [Google Scholar] [CrossRef] [PubMed]
- Debatisse, M.; Le Tallec, B.; Letessier, A.; Dutrillaux, B.; Brison, O. Common fragile sites: Mechanisms of instability revisited. Trends Genet. 2012, 28, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.-C.; Mayer, B.; Stemmann, O.; Nigg, E.A. Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J. Cell Sci. 2010, 123, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Sumner, A.T. The distribution of topoisomerase II on mammalian chromosomes. Chromosom. Res. 1996, 4, 5–14. [Google Scholar] [CrossRef]
- Díaz-Martínez, L.A.; Giménez-Abián, J.F.; Azuma, Y.; Guacci, V.; Giménez-Martín, G.; Lanier, L.M.; Clarke, D.J. PIASγ Is Required for Faithful Chromosome Segregation in Human Cells. PLoS ONE 2006, 1, e53. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.F.; Huttner, D.; Bizard, A.H.; Hirano, S.; Li, T.-N.; Palmai-Pallag, T.; Bjerregaard, V.A.; Liu, Y.; Nigg, E.A.; Wang, L.H.-C.; et al. PICH promotes sister chromatid disjunction and co-operates with topoisomerase II in mitosis. Nat. Commun. 2015, 6, 8962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kschonsak, M.; Haering, C.H. Shaping mitotic chromosomes: From classical concepts to molecular mechanisms. Bioessays 2015, 37, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Piskadlo, E.; Oliveira, R.A. Novel insights into mitotic chromosome condensation. F1000Res. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.R.; Laemmli, U.K. The structure of histone-depleted metaphase chromosomes. Cell 1977, 12, 817–828. [Google Scholar] [CrossRef]
- Adolph, K.W.; Cheng, S.M.; Laemmli, U.K. Role of nonhistone proteins in metaphase chromosome structure. Cell 1977, 12, 805–816. [Google Scholar] [CrossRef]
- Earnshaw, W.C. Architecture of metaphase chromosomes and chromosome scaffolds. J. Cell Biol. 1983, 96, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Gasser, S.M.; Laroche, T.; Falquet, J.; Boy de la Tour, E.; Laemmli, U.K. Metaphase chromosome structure. J. Mol. Biol. 1986, 188, 613–629. [Google Scholar] [CrossRef]
- Earnshaw, W.C. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J. Cell Biol. 1985, 100, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Belmont, A.S. Mitotic chromosome scaffold structure: New approaches to an old controversy. Proc. Natl. Acad. Sci. USA 2002, 99, 15855–15857. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.O. Dynamics of human {DNA} topoisomerases {IIalpha} and {IIbeta} in living cells. J. Cell Biol. 2002, 157, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Gerlich, D.; Hirota, T.; Koch, B.; Peters, J.-M.; Ellenberg, J. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr. Biol. 2006, 16, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Heidmann, S.; Sunkel, C.E. Condensin I binds chromatin early in prophase and displays a highly dynamic association with Drosophila mitotic chromosomes. Chromosoma 2007, 116, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Tavormina, P.A.; Côme, M.-G.; Hudson, J.R.; Mo, Y.-Y.; Beck, W.T.; Gorbsky, G.J. Rapid exchange of mammalian topoisomerase IIα at kinetochores and chromosome arms in mitosis. J. Cell Biol. 2002, 158, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Buchenau, P.; Saumweber, H.; Arndt-Jovin, D.J. Consequences of topoisomerase II inhibition in early embryogenesis of Drosophila revealed by in vivo confocal laser scanning microscopy. J. Cell Sci. 1993, 104, 1175–1185. [Google Scholar] [PubMed]
- Andoh, T.; Sato, M.; Narita, T.; Ishida, R. Role of DNA topoisomerase II in chromosome dynamics in mammalian cells. Biotechnol. Appl. Biochem. 1993, 18, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gorbsky, G.J. Cell cycle progression and chromosome segregation in mammalian cells cultured in the presence of the topoisomerase II inhibitors ICRF-187 [(+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane; ADR-529] and ICRF-159 (Razoxane). Cancer Res. 1994, 54, 1042–1048. [Google Scholar] [PubMed]
- Roca, J.; Ishida, R.; Berger, J.M.; Andoh, T.; Wang, J.C. Antitumor bisdioxopiperazines inhibit yeast DNA topoisomerase II by trapping the enzyme in the form of a closed protein clamp. Proc. Natl. Acad. Sci. USA 1994, 91, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.; Roberge, M. Topoisomerase II inhibitors affect entry into mitosis and chromosome condensation in BHK cells. Cell Growth Differ. 1996, 7, 83–90. [Google Scholar] [PubMed]
- Tanabe, K.; Ikegami, Y.; Ishida, R.; Andoh, T. Inhibition of Topoisomerase II by Antitumor Agents Bis(2,6-dioxopiperazine) Derivatives. Cancer Res. 1991, 51, 4903–4908. [Google Scholar] [PubMed]
- Chen, G.L.; Yang, L.; Rowe, T.C.; Halligan, B.D.; Tewey, K.M.; Liu, L.F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J. Biol. Chem. 1984, 259, 13560–13566. [Google Scholar] [PubMed]
- Fasulo, B.; Koyama, C.; Yu, K.R.; Homola, E.M.; Hsieh, T.S.; Campbell, S.D.; Sullivan, W. Chk1 and Wee1 kinases coordinate DNA replication, chromosome condensation, and anaphase entry. Mol. Biol. Cell 2012, 23, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, B.D.; Hogan, E.; Koshland, D. In vivo dissection of the chromosome condensation machinery: Reversibility of condensation distinguishes contributions of condensin and cohesin. J. Cell Biol. 2002, 156, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Vas, A.C.J.; Andrews, C.A.; Kirkland Matesky, K.; Clarke, D.J. In Vivo Analysis of Chromosome Condensation in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Petrova, B.; Dehler, S.; Kruitwagen, T.; Hériché, J.-K.; Miura, K.; Haering, C.H. Quantitative analysis of chromosome condensation in fission yeast. Mol. Cell. Biol. 2013, 33, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Ladouceur, A.-M.; Ranjan, R.; Smith, L.; Fadero, T.; Heppert, J.; Goldstein, B.; Maddox, A.S.; Maddox, P.S. CENP-A and topoisomerase-II antagonistically affect chromosome length. J. Cell Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mengoli, V.; Bucciarelli, E.; Lattao, R.; Piergentili, R.; Gatti, M.; Bonaccorsi, S. The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Drosophila Chromosome Structure. PLoS Genet. 2014, 10, e1004739. [Google Scholar] [CrossRef] [PubMed]
- Somma, M.P.; Ceprani, F.; Bucciarelli, E.; Naim, V.; De Arcangelis, V.; Piergentili, R.; Palena, A.; Ciapponi, L.; Giansanti, M.G.; Pellacani, C.; et al. Identification of Drosophila Mitotic Genes by Combining Co-Expression Analysis and RNA Interference. PLoS Genet. 2008, 4, e1000126. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Goulding, S.; Earnshaw, W.C.; Carmena, M. RNAi analysis reveals an unexpected role for topoisomerase II in chromosome arm congression to a metaphase plate. J. Cell Sci. 2003, 116, 4715–4726. [Google Scholar] [CrossRef] [PubMed]
- Samejima, K.; Samejima, I.; Vagnarelli, P.; Ogawa, H.; Vargiu, G.; Kelly, D.A.; de Lima Alves, F.; Kerr, A.; Green, L.C.; Hudson, D.F.; et al. Mitotic chromosomes are compacted laterally by KIF4 and condensin and axially by topoisomerase IIα. J. Cell Biol. 2012, 199, 755–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.; Phua, H.H.; Bennett, S.C.; Spence, J.M.; Farr, C.J. Studying vertebrate topoisomerase 2 function using a conditional knockdown system in DT40 cells. Nucleic Acids Res. 2009, 37, e98. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, A.; Kikuchi, A. Functional compatibility between isoform α and β of type II DNA topoisomerase. J. Cell Sci. 2004, 117, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.E.; Lim, C.-U.; Cole, K.; Bianchini, C.H.; Schools, G.P.; Davis, B.E.; Wada, I.; Roninson, I.B.; Broude, E. V Effects of conditional depletion of topoisomerase II on cell cycle progression in mammalian cells. Cell Cycle 2011, 10, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.J.; Porter, A.C.G. Construction, Characterization, and Complementation of a Conditional-Lethal DNA Topoisomerase IIα Mutant Human Cell Line. Mol. Biol. Cell 2004, 15, 5700–5711. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Kalitsis, P.; Chang, T.M.; Cipetic, M.; Kim, J.H.; Marshall, O.; Turnbull, L.; Whitchurch, C.B.; Vagnarelli, P.; Samejima, K.; et al. Contrasting roles of condensin I and condensin II in mitotic chromosome formation. J. Cell Sci. 2012, 125, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Farr, C.J.; Antoniou-Kourounioti, M.; Mimmack, M.L.; Volkov, A.; Porter, A.C.G. The α isoform of topoisomerase II is required for hypercompaction of mitotic chromosomes in human cells. Nucleic Acids Res. 2014, 42, 4414–4426. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Luke, M.; Laemmli, U.K. Chromosome assembly in vitro: Topoisomerase II is required for condensation. Cell 1991, 64, 137–148. [Google Scholar] [CrossRef]
- Hirano, T.; Mitchison, J.T. Topoisomerase II does not play a scaffolding role in the organization of mitotic chromosomes assembled in Xenopus egg extracts. J. Cell Biol. 1993, 120, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Shintomi, K.; Takahashi, T.S.; Hirano, T. Reconstitution of mitotic chromatids with a minimum set of purified factors. Nat. Cell Biol. 2015, 17, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Deming, P.B.; Cistulli, C.A.; Zhao, H.; Graves, P.R.; Piwnica-Worms, H.; Paules, R.S.; Downes, C.S.; Kaufmann, W.K. The human decatenation checkpoint. Proc. Natl. Acad. Sci. USA 2001, 98, 12044–12049. [Google Scholar] [CrossRef] [PubMed]
- Roberge, M.; Th’ng, J.; Hamaguchi, J.; Bradbury, E.M. The topoisomerase II inhibitor VM-26 induces marked changes in histone H1 kinase activity, histones H1 and H3 phosphorylation, and chromosome condensation in G2 phase and mitotic BHK cells. J. Cell Biol. 1990, 111, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, P.R.; Lacroix, F.B.; Margolis, R.L. Chromosomes with Two Intact Axial Cores Are Induced by G(2 )Checkpoint Override: Evidence That DNA Decatenation Is not Required to Template the Chromosome Structure. J. Cell Biol. 1997, 136, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Azuma, Y. Non-Catalytic Roles of the Topoisomerase IIα C-Terminal Domain. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.O.; Larsen, M.K.; Barthelmes, H.U.; Hock, R.; Andersen, C.L.; Kjeldsen, E.; Knudsen, B.R.; Westergaard, O.; Boege, F.; Mielke, C. Dynamics of human DNA topoisomerases IIalpha and IIbeta in living cells. J. Cell Biol. 2002, 157, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Long, B.H. Mechanisms of action of teniposide (VM-26) and comparison with etoposide (VP-16). Semin. Oncol. 1992, 19, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, R.; Pope, L.H.; Christensen, M.O.; Sun, M.; Terekhova, K.; Boege, F.; Mielke, C.; Andersen, A.H.; Marko, J.F. Mitotic chromosomes are constrained by topoisomerase II–sensitive DNA entanglements. J. Cell Biol. 2010, 188, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Liu, C.-C.; Alberts, B.M. Type II DNA topoisomerases: Enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell 1980, 19, 697–707. [Google Scholar] [CrossRef]
- Hsieh, T. Knotting of the circular duplex DNA by type II DNA topoisomerase from Drosophila melanogaster. J. Biol. Chem. 1983, 258, 8413–8420. [Google Scholar] [PubMed]
- Roca, J.; Berger, J.M.; Wang, J.C. On the simultaneous binding of eukaryotic DNA topoisomerase II to a pair of double-stranded DNA helices. J. Biol. Chem. 1993, 268, 14250–14255. [Google Scholar] [PubMed]
- Valdés, A.; Segura, J.; Dyson, S.; Martínez-García, B.; Roca, J. DNA knots occur in intracellular chromatin. Nucleic Acids Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Maresca, T.J.; Salmon, E.D. Welcome to a new kind of tension: Translating kinetochore mechanics into a wait-anaphase signal. J. Cell Sci. 2010, 123, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Khodjakov, A.; Pines, J. Centromere tension: A divisive issue. Nat. Cell Biol. 2010, 12, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.A.; Vas, A.C.; Meier, B.; Giménez-Abián, J.F.; Díaz-Martínez, L.A.; Green, J.; Erickson, S.L.; VanderWaal, K.E.; Hsu, W.-S.; Clarke, D.J. A mitotic topoisomerase II checkpoint in budding yeast is required for genome stability but acts independently of Pds1/securin. Genes Dev. 2006, 20, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Skoufias, D.A.; Lacroix, F.B.; Andreassen, P.R.; Wilson, L.; Margolis, R.L. Inhibition of DNA Decatenation, but Not DNA Damage, Arrests Cells at Metaphase. Mol. Cell 2004, 15, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, A.; Shinohara, M.; Rieder, C.L. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J. Cell Biol. 2004, 166, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; Vas, A.C.; Andrews, C.A.; Díaz-Martínez, L.A.; Gimenez-Abian, J.F. Topoisomerase II Checkpoints: Universal Mechanisms that Regulate Mitosis. Cell Cycle 2006, 5, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Warsi, T.H.; Navarro, M.S.; Bachant, J. DNA Topoisomerase II Is a Determinant of the Tensile Properties of Yeast Centromeric Chromatin and the Tension Checkpoint. Mol. Biol. Cell 2008, 19, 4421–4433. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.H.; Fernald, A.W.; Osheroff, N. Protein kinase C modulates the catalytic activity of topoisomerase II by enhancing the rate of ATP hydrolysis: Evidence for a common mechanism of regulation by phosphorylation. Biochemistry 1993, 32, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Chikamori, K.; Grabowski, D.R.; Kinter, M.; Willard, B.B.; Yadav, S.; Aebersold, R.H.; Bukowski, R.M.; Hickson, I.D.; Andersen, A.H.; Ganapathi, R.; et al. Phosphorylation of Serine 1106 in the Catalytic Domain of Topoisomerase IIα Regulates Enzymatic Activity and Drug Sensitivity. J. Biol. Chem. 2003, 278, 12696–12702. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Furuta, M.; Kirkpatrick, D.; Gygi, S.P.; Azuma, Y. PIASy-dependent SUMOylation regulates DNA topoisomerase IIα activity. J. Cell Biol. 2010, 191, 783–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, Y.; Arnaoutov, A.; Anan, T.; Dasso, M. PIASy mediates SUMO-2 conjugation of Topoisomerase-II on mitotic chromosomes. EMBO J. 2005, 24, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, M.; Santos, V.; Ferreira, F.; Costa, R.; Cardoso, J.; Pinheiro, I.; Rino, J.; Jaffray, E.; Hay, R.T.; Ferreira, J. Conjugation of Human Topoisomerase 2α with Small Ubiquitin-like Modifiers 2/3 in Response to Topoisomerase Inhibitors: Cell Cycle Stage and Chromosome Domain Specificity. Cancer Res. 2008, 68, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Dawlaty, M.M.; Malureanu, L.; Jeganathan, K.B.; Kao, E.; Sustmann, C.; Tahk, S.; Shuai, K.; Grosschedl, R.; van Deursen, J.M. Resolution of Sister Centromeres Requires RanBP2-Mediated SUMOylation of Topoisomerase IIα. Cell 2008, 133, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yong-Gonzalez, V.; Kikuchi, Y.; Strunnikov, A. SIZ1/SIZ2 Control of Chromosome Transmission Fidelity Is Mediated by the Sumoylation of Topoisomerase II. Genetics 2006, 172, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deibler, R.W.; Chan, H.S.; Zechiedrich, L. The why and how of DNA unlinking. Nucleic Acids Res. 2009, 37, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Rybenkov, V.V.; Ullsperger, C.; Vologodskii, A.V.; Cozzarelli, N.R. Simplification of DNA Topology Below Equilibrium Values by Type II Topoisomerases. Science 1997, 277, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 2006, 7, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, F. SMC complexes: From DNA to chromosomes. Nat. Rev. Mol. Cell Biol. 2016, 17, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Rybenkov, V.V.; Herrera, V.; Petrushenko, Z.M.; Zhao, H. MukBEF, a chromosomal organizer. J. Mol. Microbiol. Biotechnol. 2014, 24, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Stewart, N.K.; Berger, A.J.; Vos, S.; Schoeffler, A.J.; Berger, J.M.; Chait, B.T.; Oakley, M.G. Escherichia coli condensin MukB stimulates topoisomerase IV activity by a direct physical interaction. Proc. Natl. Acad. Sci. USA 2010, 107, 18832–18837. [Google Scholar] [CrossRef] [PubMed]
- Hayama, R.; Marians, K.J. Physical and functional interaction between the condensin MukB and the decatenase topoisomerase IV in Escherichia coli. Proc. Natl. Acad. Sci. USA 2010, 107, 18826–18831. [Google Scholar] [CrossRef] [PubMed]
- Hayama, R.; Bahng, S.; Karasu, M.E.; Marians, K.J. The MukB-ParC Interaction Affects the Intramolecular, Not Intermolecular, Activities of Topoisomerase IV. J. Biol. Chem. 2013, 288, 7653–7661. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, E.; Upton, A.L.; Uphoff, S.; Henry, O.; Badrinarayanan, A.; Sherratt, D. The SMC Complex MukBEF Recruits Topoisomerase IV to the Origin of Replication Region in Live Escherichia coli. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, P.; Stracy, M.; Ginda, K.; Zawadzka, K.; Lesterlin, C.; Kapanidis, A.N.; Sherratt, D.J. The Localization and Action of Topoisomerase IV in Escherichia coli Chromosome Segregation Is Coordinated by the SMC Complex, MukBEF. Cell Rep. 2015, 13, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nurse, P.; Bahng, S.; Lee, C.M.; Marians, K.J. The MukB–topoisomerase IV interaction is required for proper chromosome compaction. J. Biol. Chem. 2017, 292, 16921–16932. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, O.W.; Riley, E.P.; Rudner, D.Z. The SMC Condensin Complex Is Required for Origin Segregation in Bacillus subtilis. Curr. Biol. 2014, 24, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Mascarenhas, J.; Kösters, B.; Hasilik, A.; Graumann, P.L. Genetic interaction of the SMC complex with topoisomerase IV in Bacillus subtilis. Microbiology 2005, 151, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, K.; Kanno, T.; Shirahige, K.; Sjogren, C. The maintenance of chromosome structure: Positioning and functioning of SMC complexes. Nat. Rev. Mol. Cell Biol. 2014, 15, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Berta, D.G.; Sjögren, C. The Smc5/6 Complex Is an ATP-Dependent Intermolecular DNA Linker. Cell Rep. 2015, 12, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, K.; Carlborg, K.K.; Nakato, R.; Berta, D.G.; Lilienthal, I.; Kanno, T.; Lindqvist, A.; Brink, M.C.; Dantuma, N.P.; Katou, Y.; et al. The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement. PLoS Genet. 2014, 10, e1004680. [Google Scholar] [CrossRef] [PubMed]
- Verver, D.E.; Zheng, Y.; Speijer, D.; Hoebe, R.; Dekker, H.L.; Repping, S.; Stap, J.; Hamer, G. Non-SMC Element 2 (NSMCE2) of the SMC5/6 Complex Helps to Resolve Topological Stress. Int. J. Mol. Sci. 2016, 17, 1782. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Paez, L.M.; Tanaka, H.; Bando, M.; Takahashi, M.; Nozaki, N.; Nakato, R.; Shirahige, K.; Hirota, T. Smc5/6-mediated regulation of replication progression contributes to chromosome assembly during mitosis in human cells. Mol. Biol. Cell 2014, 25, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Pryzhkova, M.V.; Jordan, P.W. Conditional mutation of Smc5 in mouse embryonic stem cells perturbs condensin localization and mitotic progression. J. Cell Sci. 2016, 129, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Jordan, P.W.; Viera, A.; Alsheimer, M.; Fukuda, T.; Jessberger, R.; Llano, E.; Pendás, A.M.; Handel, M.A.; Suja, J.A. Dynamic localization of SMC5/6 complex proteins during mammalian meiosis and mitosis suggests functions in distinct chromosome processes. J. Cell Sci. 2013, 126, 4239–4252. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, M.; Oliveira, R.A. Centromeric Cohesin: Molecular Glue and Much More. In Centromeres and Kinetochores; Black, B.E., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 485–513. ISBN 978-3-319-58592-5. [Google Scholar]
- Litwin, I.; Wysocki, R. New insights into cohesin loading. Curr. Genet. 2017. [Google Scholar] [CrossRef] [PubMed]
- Haarhuis, J.H.I.; Elbatsh, A.M.O.; Rowland, B.D. Cohesin and Its Regulation: On the Logic of X-Shaped Chromosomes. Dev. Cell 2014, 31, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Farcas, A.-M.; Uluocak, P.; Helmhart, W.; Nasmyth, K. Cohesin’s Concatenation of Sister DNAs Maintains Their Intertwining. Mol. Cell 2011, 44, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Viera, A.; Berenguer, I.; Llano, E.; Pendás, A.M.; Barbero, J.L.; Kikuchi, A.; Suja, J.A. Cohesin removal precedes topoisomerase IIα-dependent decatenation at centromeres in male mammalian meiosis II. Chromosoma 2014, 123, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Wutz, G.; Huet, S.; Jaritz, M.; Wuensche, A.; Schirghuber, E.; Davidson, I.F.; Tang, W.; Cisneros, D.A.; Bhaskara, V.; et al. Wapl is an essential regulator of chromatin structure and chromosome segregation. Nature 2013, 501, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Haarhuis, J.H.I.; Elbatsh, A.M.O.; van den Broek, B.; Camps, D.; Erkan, H.; Jalink, K.; Medema, R.H.; Rowland, B.D. WAPL-Mediated Removal of Cohesin Protects against Segregation Errors and Aneuploidy. Curr. Biol. 2013, 23, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Kotadia, S.; Tavares, A.; Mirkovic, M.; Bowlin, K.; Eichinger, C.S.; Nasmyth, K.; Sullivan, W. Centromere-Independent Accumulation of Cohesin at Ectopic Heterochromatin Sites Induces Chromosome Stretching during Anaphase. PLoS Biol. 2014, 12, e1001962. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W.; Szostak, J.W. Chromosome Segregation in Mitosis and Meiosis. Annu. Rev. Cell Biol. 1985, 1, 289–315. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, F.; Wernic, D.; Poupart, M.-A.; Koonin, E.V.; Nasmyth, K. Cleavage of Cohesin by the CD Clan Protease Separin Triggers Anaphase in Yeast. Cell 2000, 103, 375–386. [Google Scholar] [CrossRef]
- Díaz-Martínez, L.A.; Giménez-Abián, J.F.; Clarke, D.J. Chromosome cohesion–Rings, knots, orcs and fellowship. J. Cell Sci. 2008, 121, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Yanagida, M. Coordinated Requirements of Human Topo II and Cohesin for Metaphase Centromere Alignment under Mad2-dependent Spindle Checkpoint Surveillance. Mol. Biol. Cell 2006, 17, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Vagnarelli, P.; Morrison, C.; Dodson, H.; Sonoda, E.; Takeda, S.; Earnshaw, W.C. Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores. EMBO Rep. 2004, 5, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Dewar, H.; Tanaka, K.; Nasmyth, K.; Tanaka, T.U. Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature 2004, 428, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Condensin-Based Chromosome Organization from Bacteria to Vertebrates. Cell 2016, 164, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Mitchison, T.J. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell 1994, 79, 449–458. [Google Scholar] [CrossRef]
- Hirano, T.; Kobayashi, R.; Hirano, M. Condensins, Chromosome Condensation Protein Complexes Containing XCAP-C, XCAP-E and a Xenopus Homolog of the Drosophila Barren Protein. Cell 1997, 89, 511–521. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Coelho, P.A.; Sunkel, C.E. The Condensin I Subunit Barren/CAP-H Is Essential for the Structural Integrity of Centromeric Heterochromatin during Mitosis. Mol. Cell. Biol. 2005, 25, 8971–8984. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.A.; Gatlin, J.C.; Dong, Y.; Joglekar, A.; Cameron, L.; Hudson, D.F.; Farr, C.J.; Mcewen, B.F.; Salmon, E.D.; Earnshaw, W.C.; et al. Condensin Regulates the Stiffness of Vertebrate Centromeres. Mol. Biol. Cell 2009, 20, 2371–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, D.F.; Vagnarelli, P.; Gassmann, R.; Earnshaw, W.C. Condensin Is Required for Nonhistone Protein Assembly and Structural Integrity of Vertebrate Mitotic Chromosomes. Dev. Cell 2003, 5, 323–336. [Google Scholar] [CrossRef]
- Bhat, M.A.; Philp, A.V.; Glover, D.M.; Bellen, H.J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with Topoisomerase II. Cell 1996, 87, 1103–1114. [Google Scholar] [CrossRef]
- Steffensen, S.; Coelho, P.A.; Cobbe, N.; Vass, S.; Costa, M.; Hassan, B.; Prokopenko, S.N.; Bellen, H.; Heck, M.M.S.; Sunkel, C.E. A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr. Biol. 2001, 11, 295–307. [Google Scholar] [CrossRef]
- Hagstrom, K.A. C. elegans condensin promotes mitotic chromosome architecture centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 2002, 16, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.A.; Queiroz-Machado, J.; Sunkel, C.E. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J. Cell Sci. 2003, 116, 4763–4776. [Google Scholar] [CrossRef] [PubMed]
- Cuvier, O.; Hirano, T. A role of topoisomerase II in linking DNA replication to chromosome condensation. J. Cell Biol. 2003, 160, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.; Aragón, L. A model for chromosome condensation based on the interplay between condensin and topoisomerase II. Trends Genet. 2012, 28, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hirano, T. ATP-Dependent Positive Supercoiling of DNA by 13S Condensin: A Biochemical Implication for Chromosome Condensation. Cell 1997, 90, 625–634. [Google Scholar] [CrossRef]
- Kimura, K.; Hirano, T. Dual roles of the 11S regulatory subcomplex in condensin functions. Proc. Natl. Acad. Sci. USA 2000, 97, 11972–11977. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Cuvier, O.; Hirano, T. Chromosome Condensation by a Human Condensin Complex in Xenopus Egg Extracts. J. Biol. Chem. 2001, 276, 5417–5420. [Google Scholar] [CrossRef] [PubMed]
- Eeftens, J.M.; Bisht, S.; Kschonsak, M.; Haering, C.H.; Dekker, C. Real-time detection of condensin-driven DNA compaction reveals a multistep binding mechanism. EMBO J. 2017, 36, 3269–3405. [Google Scholar] [CrossRef] [PubMed]
- Nasmyth, K. Disseminating the Genome: Joining, Resolving, and Separating Sister Chromatids During Mitosis and Meiosis. Annu. Rev. Genet. 2001, 35, 673–745. [Google Scholar] [CrossRef] [PubMed]
- Alipour, E.; Marko, J.F. Self-organization of domain structures by DNA-loop-extruding enzymes. Nucleic Acids Res. 2012, 40, 11202–11212. [Google Scholar] [CrossRef] [PubMed]
- Goloborodko, A.; Imakaev, M.V.; Marko, J.F.; Mirny, L. Compaction and segregation of sister chromatids via active loop extrusion. Elife 2016, 5, e14864. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, T.; Bisht, S.; Eeftens, J.M.; Dekker, C.; Haering, C.H.; Greene, E.C. The condensin complex is a mechanochemical motor that translocates along DNA. Science 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Brandão, H.B.; Le, T.B.K.; Laub, M.T.; Rudner, D.Z. Bacillus subtilis SMC complexes juxtapose chromosome arms as they travel from origin to terminus. Science 2017, 355, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Diebold-Durand, M.-L.; Lee, H.; Ruiz Avila, L.B.; Noh, H.; Shin, H.-C.; Im, H.; Bock, F.P.; Bürmann, F.; Durand, A.; Basfeld, A.; et al. Structure of Full-Length SMC and Rearrangements Required for Chromosome Organization. Mol. Cell 2017, 67, 334.e5–347.e5. [Google Scholar] [CrossRef] [PubMed]
- Shintomi, K.; Hirano, T. The relative ratio of condensin I to II determines chromosome shapes. Genes Dev. 2011, 25, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Elbatsh, A.M.O.; Raaijmakers, J.A.; van der Weide, R.H.; uit de Bos, J.; Teunissen, H.; Bravo, S.; Medema, R.H.; de Wit, E.; Haering, C.H.; Rowland, B.D. Condensin’s ATPase Machinery Drives and Dampens Mitotic Chromosome Condensation. bioRxiv 2017. [Google Scholar] [CrossRef]
- Houlard, M.; Godwin, J.; Metson, J.; Lee, J.; Hirano, T.; Nasmyth, K. Condensin confers the longitudinal rigidity of chromosomes. Nat. Cell Biol. 2015, 17, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Mazia, D. Mitosis and the physiology of cell division. Cell. Biochem. Physiol. Morphol. 1961, 77–412. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piskadlo, E.; Oliveira, R.A. A Topology-Centric View on Mitotic Chromosome Architecture. Int. J. Mol. Sci. 2017, 18, 2751. https://doi.org/10.3390/ijms18122751
Piskadlo E, Oliveira RA. A Topology-Centric View on Mitotic Chromosome Architecture. International Journal of Molecular Sciences. 2017; 18(12):2751. https://doi.org/10.3390/ijms18122751
Chicago/Turabian StylePiskadlo, Ewa, and Raquel A. Oliveira. 2017. "A Topology-Centric View on Mitotic Chromosome Architecture" International Journal of Molecular Sciences 18, no. 12: 2751. https://doi.org/10.3390/ijms18122751