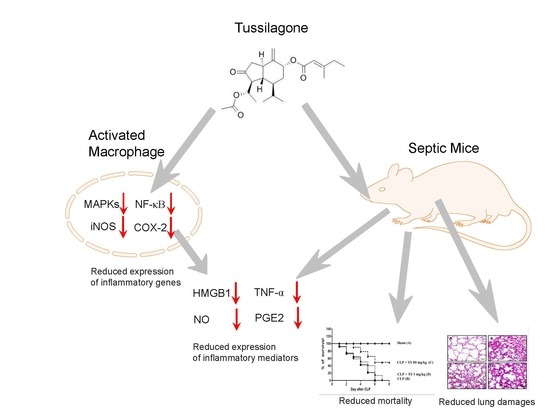
Tussilagone Inhibits the Inflammatory Response and Improves Survival in CLP-Induced Septic Mice
Abstract
:1. Introduction
2. Results
2.1. TS Inhibits the Production of NOs and PGE2 in LPS-Stimulated Macrophages
2.2. TS Inhibits TNF-α and HMGB1 Expression in LPS-Stimulated Macrophages
2.3. TS Exerts Anti-Inflammatory Effects in LPS-Stimulated Peritoneal Macrophages
2.4. TS Suppresses MAP Kinase Activation
2.5. TS Inhibits the LPS-Mediated Activation of NF-κB in RAW 264.7 Cells
2.6. TS Improves Survival during Cecal Ligation and Puncture (CLP)-Induced Sepsis
2.7. TS Suppresses the Serum Levels of Inflammatory Mediators in Septic Mice
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Purification and Analysis of TS
4.3. Cell Viability Assay
4.4. Colorimetric Determination of NO
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Western Blot Analysis
4.7. Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.8. Isolation of Peritoneal Macrophages and Alveola Macrophages
4.9. Transient Transfection and Luciferase Assay
4.10. Preparation of Nuclear Extract
4.11. Animals
4.12. Sepsis Model and Effects of Tussilagone
4.13. Organ Injury Experiments
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CLP | cecal ligation and puncture |
| DMSO | dimethyl sulfoxide |
| ELISA | enzyme-linked immunosorbent assay |
| ERK | extracellular-signal-regulated kinase |
| FBS | fetal bovine serum |
| HMGB1 | high-mobility group box 1 |
| HO-1 | heme oxygenase-1 |
| HPLC | high-performance liquid chromatography |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharide |
| NO | nitric oxide |
| Nrf2 | NF-E2-related factor 2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| MAP | mitogen-activated protein |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PAM | pulmonary alveolar macrophages |
| PGE2 | Prostaglandin E2 |
| PCR | polymerase chain reaction |
| TNF-α | tumor necrosis factor-alpha |
| TS | tussilagone |
References
- Esposito, S.; De Simone, G.; Boccia, G.; De Caro, F.; Pagliano, P. Sepsis and septic shock: New definitions, new diagnostic and therapeutic approaches. J. Glob. Antimicrob. Resist. 2017, 10, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Suarez De La Rica, A.; Gilsanz, F.; Maseda, E. Epidemiologic trends of sepsis in western countries. Ann. Transl. Med. 2016, 4, 325. [Google Scholar] [CrossRef] [PubMed]
- Bellingan, G. Inflammatory cell activation in sepsis. Br. Med. Bull. 1999, 55, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, L.; Tracey, K.J. The “cytokine profile”: A code for sepsis. Trends Mol. Med. 2005, 11, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.K.; Chiang, E.T.; Garcia, J.G. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc. Res. 2011, 81, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Barnay-Verdier, S.; Fattoum, L.; Borde, C.; Kaveri, S.; Gibot, S.; Marechal, V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 2011, 37, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Venereau, E.; De Leo, F.; Mezzapelle, R.; Careccia, G.; Musco, G.; Bianchi, M.E. HMGB1 as biomarker and drug target. Pharmacol. Res. 2016, 111, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Lee, H.S.; Ryu, J.H. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression by tussilagone from Farfarae flos in BV-2 microglial cells. Arch. Pharm. Res. 2008, 31, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, C.; Lee, H.S.; Park, J.; Choe, J.; Lee, J.H. The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 2009, 9, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ryu, H.S.; Lee, H.K.; Kim, J.S.; Yun, J.; Kang, J.S.; Hwang, B.Y.; Hong, J.T.; Kim, Y.; Han, S.B. Tussilagone inhibits dendritic cell functions via induction of heme oxygenase-1. Int. Immunopharmacol. 2014, 22, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, Y.; Zhao, T.M.; Zhang, Q. Preparation of Sesquiterpenoids from Tussilago farfara L. by High-speed Counter-current Chromatography. Pharmacogn. Mag. 2016, 12, 282–287. [Google Scholar] [PubMed]
- Yu, F.; Utsumi, R. Diversity, regulation, and genetic manipulation of plant mono- and sesquiterpenoid biosynthesis. Cell. Mol. Life Sci. 2009, 66, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Little, D.B.; Croteau, R.B. Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases delta-selinene synthase and gamma-humulene synthase. Arch. Biochem. Biophys. 2002, 402, 120–135. [Google Scholar] [CrossRef]
- Schnee, C.; Kollner, T.G.; Gershenzon, J.; Degenhardt, J. The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-beta-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol. 2002, 130, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Qureshi, M.Z.; Attar, R.; Aslam, A.; Kanwal, S.; Khalid, S.; Qureshi, J.M.; Aras Perk, A.; Farooqi, A.A.; Ismail, M. How sesquiterpenes modulate signaling cascades in cancers. Cell. Mol. Biol. 2016, 62, 110–117. [Google Scholar] [PubMed]
- da Silveira e Sa Rde, C.; Andrade, L.N.; de Sousa, D.P. Sesquiterpenes from Essential Oils and Anti-Inflammatory Activity. Nat. Prod. Commun. 2015, 10, 1767–1774. [Google Scholar] [PubMed]
- Repetto, M.G.; Boveris, A. Bioactivity of sesquiterpenes: Compounds that protect from alcohol-induced gastric mucosal lesions and oxidative damage. Mini Rev. Med. Chem. 2010, 10, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, A.; Sur, S.; Kumar, S.K.; Khan, S.R. Sesquiterpenes: Natural products that decrease cancer growth. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 477–499. [Google Scholar] [CrossRef]
- Lee, J.; Kang, U.; Seo, E.K.; Kim, Y.S. Heme oxygenase-1-mediated anti-inflammatory effects of tussilagonone on macrophages and 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Int. Immunopharmacol. 2016, 34, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Jiang, H. Nrf-2-HO-1-HMGB1 axis: An important therapeutic approach for protection against myocardial ischemia and reperfusion injury. Int. J. Cardiol. 2014, 172, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ward, M.F.; Sama, A.E. Targeting HMGB1 in the treatment of sepsis. Expert Opin. Ther. Targets 2014, 18, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.H.; Baek, S.H.; Lee, H.J.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-κB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochem. Pharmacol. 2006, 71, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Hevia, H.; Varela-Rey, M.; Corrales, F.J.; Berasain, C.; Martinez-Chantar, M.L.; Latasa, M.U.; Lu, S.C.; Mato, J.M.; Garcia-Trevijano, E.R.; Avila, M.A. 5′-methylthioadenosine modulates the inflammatory response to endotoxin in mice and in rat hepatocytes. Hepatology 2004, 39, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.J.; Wang, X.; Wancket, L.M.; Li, X.A.; Meng, X.; Nelin, L.D.; Cato, A.C.; Liu, Y. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J. Immunol. 2009, 183, 7411–7419. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.N.; Zong, Y.; Zhong, L.M.; Li, Y.M.; Zhang, W.; Bian, L.G.; Ai, Q.L.; Liu, Y.D.; Sun, J.; Lu, D. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS ONE 2011, 6, e21891. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Kim, Y.K.; Hong, S.B.; Lee, K.J. Naringin decreases TNF-α and HMGB1 release from LPS-stimulated macrophages and improves survival in a CLP-induced sepsis mice. PLoS ONE 2016, 11, e0164186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Nie, Y.C.; Chen, J.L.; Su, W.W.; Li, P.B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. Int. Immunopharmacol. 2011, 11, 1606–1612. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.K.; Yeo, M.G.; Oh, B.K.; Kim, H.Y.; Yang, H.J.; Cho, S.-S.; Gil, M.; Lee, K.J. Tussilagone Inhibits the Inflammatory Response and Improves Survival in CLP-Induced Septic Mice. Int. J. Mol. Sci. 2017, 18, 2744. https://doi.org/10.3390/ijms18122744
Kim YK, Yeo MG, Oh BK, Kim HY, Yang HJ, Cho S-S, Gil M, Lee KJ. Tussilagone Inhibits the Inflammatory Response and Improves Survival in CLP-Induced Septic Mice. International Journal of Molecular Sciences. 2017; 18(12):2744. https://doi.org/10.3390/ijms18122744
Chicago/Turabian StyleKim, Yun Kyu, Myeong Gu Yeo, Bo Kang Oh, Ha Yeong Kim, Hun Ji Yang, Seung-Sik Cho, Minchan Gil, and Kyung Jin Lee. 2017. "Tussilagone Inhibits the Inflammatory Response and Improves Survival in CLP-Induced Septic Mice" International Journal of Molecular Sciences 18, no. 12: 2744. https://doi.org/10.3390/ijms18122744