Ulmus macrocarpa Hance Extracts Attenuated H2O2 and UVB-Induced Skin Photo-Aging by Activating Antioxidant Enzymes and Inhibiting MAPK Pathways
Abstract
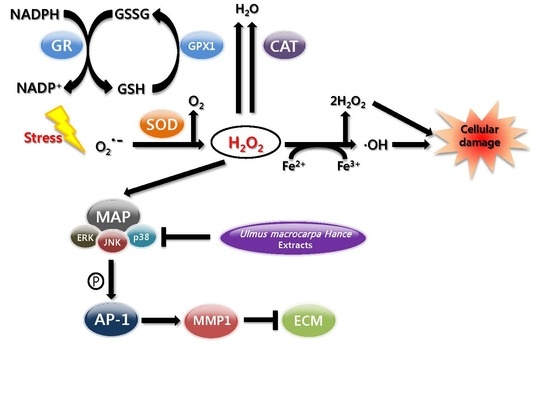
:1. Introduction
2. Results
2.1. Various Antioxidant Compounds in Ulmus macrocarpa Hance (UMH) Extracts
2.2. Antioxidant Activity of UMH Extracts
2.3. UMH Extracts Protect Human Dermal Fibroblasts (HDFs) from H2O2-Induced Cell Death
2.4. UMH Extracts Inhibit H2O2- to Induce ROS Production in HDFs
2.5. UMH Extracts Inhibit Premature Senescence Induced by Hydrogen Peroxide in HDFs
2.6. UMH on the Expression of Cellular Antioxidant Enzymes
2.7. UMH Blockade of c-Jun N-Terminal Kinase (JNK) and p38 MAPK Signaling Pathways
2.8. UMH Inhibit UVB-Induced Photo-Aging in Skin of Hairless Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of the Phenol Compounds
4.3. Determination of the Antioxidant Activity
4.4. Cell Culture and Cell Viability
4.5. Measurement of Intracellular Reactive Oxygen Species (ROS) Generation
4.6. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
4.7. Western Blot Analysis
4.8. UV Irradiation of Hairless Mice
4.9. Wrinkle Measurement
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| SOD | Susawperoxide dismutase |
| CAT | Catalase |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
References
- Gilchrest, B.A.; Garmyn, M.; Yaar, M. Aging and photoaging affect gene expression in cultured human keratinocytes. Arch. Dermatol. 1994, 130, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Cellular and molecular mechanisms of cutaneous aging. J. Dermatol. Surg. Oncol. 1990, 16, 915–922. [Google Scholar] [CrossRef] [PubMed]
- MatÉs, J.M.; Pérez-Gómez, C.; de Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-κB, and Akt in normal and malignant human mesothelial cells. Environ. Health Persp. 2008, 116, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, A.; Psotova, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. Repub. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Kim, K.B.; Jo, B.S.; Park, H.J.; Park, K.T.; An, B.J.; Ahn, D.H.; Kim, M.U.; Chae, J.W.; Cho, Y.J. Healthy functional food properties of phenolic compounds isolated from Ulmus pumila. Korean J. Food Preserv. 2012, 19, 909–918. [Google Scholar] [CrossRef]
- Kim, D.B.; Shin, G.H.; Kim, J.M.; Kim, Y.H.; Lee, J.H.; Lee, J.S.; Song, H.J.; Choe, S.Y.; Park, I.J.; Cho, J.H.; et al. Antioxidant and anti-ageing activities of citrus-based juice mixture. Food Chem. 2016, 194, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, J.S.; Lee, K.R.; Ha, S.J.; Hong, E.K. Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence. Nutrients 2014, 6, 3711–3726. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [PubMed]
- Dasari, A.; Bartholomew, J.N.; Volonte, D.; Galbiati, F. Oxidative stress induces premature senescence by stimulating protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006, 66, 10805–10814. [Google Scholar] [CrossRef] [PubMed]
- Assche, F.; Clijsters, H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Gum, R.; Wang, H.; Lengyel, E.; Juarez, J.; Boyd, D. Regulation of 92 kDa type IV collagenase expression by the jun aminoterminal kinase- and the extracellular signal-regulated kinase dependent signaling cascades. Oncogene 1997, 14, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Darr, D.; Fridovich, I. Free radicals in cutaneous biology. J. Investig. Dermatol. 1994, 102, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Cooper, P.K.; Ganesan, A.K.; Smith, C.A. DNA repair in bacteria and mammalian cells. Ann. Rev. Biochem. 1979, 48, 783–836. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Kim, J.S.; Bae, J.O.; Yoon, H.S. Antioxidative effectiveness of water extract and ether in wormwood (Artemisia montana Pampan). J. Korean Soc. Food Sci. Nutr. 1992, 21, 17–22. [Google Scholar]
- Jeong, H.J.; Park, S.B.; Kim, S.; Kim, H.K. Total polyphenol content and antioxidative activity of wild grape (Vitis coignetiae) extracts depending on ethanol concentrations. J. Korean Soc. Food Sci. Nutr. 2007, 36, 1491–1496. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Duh, P.D. Antioxidant activity of burdock (Arctium lappa Linné): Its scavenging effect on free-radical and active oxygen. J. Am. Oil Chem. Soc. 1988, 75, 455–461. [Google Scholar] [CrossRef]
- Oyaizu, M. Antioxidative activities of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. J. Jpn. Soc. Food Sci. 1988, 35, 771–775. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Cho, M.L.; Seo, K.E.; Kim, Y.S.; Jung, T.D.; Kim, Y.H.; Kim, D.B.; Shin, G.H.; Oh, J.W.; Lee, J.S.; et al. Effect of extraction conditions on in vitro antioxidant activities of root bark extract from Ulmus pumila L. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1172–1179. [Google Scholar] [CrossRef]
- Hwang, B.M.; Noh, E.M.; Kim, J.S.; Kim, J.M.; You, Y.O.; Hwang, J.K.; Kwon, K.B.; Lee, Y.R. Curcumin inhibits UVB-induced matrix metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK pathways in human dermal fibroblasts. Exp. Dermatol. 2013, 22, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Mitsunaga, T.; Doi, T.; Kondo, Y.; Abe, I. Color development of proanthocyanidins in vanillin-hydrochloric acid reaction. J. Wood Sci. 1998, 44, 125–130. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.B.; Lee, J.S.; Cho, J.H.; Kim, B.K.; Choi, H.S.; Lee, B.Y.; Lee, O.H. Antioxidant activity and Anti adipogenic effects of wild herbs mainly cultivated in Korea. Molecules 2013, 18, 12937–12950. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Robert, R.; Nicoletta, P.; Anna, P.; Ananth, P.; Min, Y.; Catherine, R.E. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-I.; Lee, J.-H.; Kim, J.-M.; Jung, T.-D.; Cho, B.-Y.; Choi, S.-H.; Lee, D.-W.; Kim, J.; Kim, J.-Y.; Lee, O.-H. Ulmus macrocarpa Hance Extracts Attenuated H2O2 and UVB-Induced Skin Photo-Aging by Activating Antioxidant Enzymes and Inhibiting MAPK Pathways. Int. J. Mol. Sci. 2017, 18, 1200. https://doi.org/10.3390/ijms18061200
Choi S-I, Lee J-H, Kim J-M, Jung T-D, Cho B-Y, Choi S-H, Lee D-W, Kim J, Kim J-Y, Lee O-H. Ulmus macrocarpa Hance Extracts Attenuated H2O2 and UVB-Induced Skin Photo-Aging by Activating Antioxidant Enzymes and Inhibiting MAPK Pathways. International Journal of Molecular Sciences. 2017; 18(6):1200. https://doi.org/10.3390/ijms18061200
Chicago/Turabian StyleChoi, Sun-Il, Jin-Ha Lee, Jae-Min Kim, Tae-Dong Jung, Bong-Yeon Cho, Seung-Hyun Choi, Dae-Won Lee, Jinkyung Kim, Jong-Yea Kim, and Ok-Hawn Lee. 2017. "Ulmus macrocarpa Hance Extracts Attenuated H2O2 and UVB-Induced Skin Photo-Aging by Activating Antioxidant Enzymes and Inhibiting MAPK Pathways" International Journal of Molecular Sciences 18, no. 6: 1200. https://doi.org/10.3390/ijms18061200