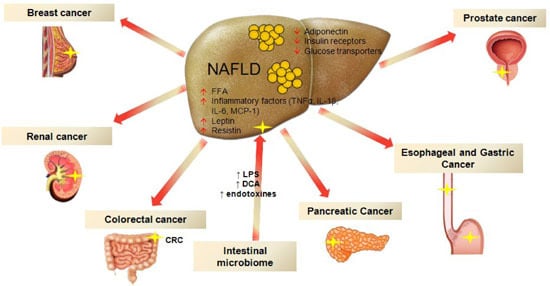
Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers
Abstract
:1. Introduction
2. Nonalcoholic Fatty Liver Disease (NAFLD) and Colorectal Cancer
3. NAFLD and Cancers in Other Sites
3.1. Esophageal and Gastric Cancer
3.2. Pancreatic Cancer
3.3. Renal Cancer
3.4. Breast Cancer
3.5. Prostate Cancer
4. Putative Role of Insulin Resistance and Gut Microbiota in the Development of Extra-Hepatic Cancers in NAFLD
5. Conclusions
Conflicts of Interest
Abbreviations
| AMPK | AMPc-activated protein kinase |
| CI | confidence interval |
| COX-2 | cyclooxygenase 2 |
| CRC | colorectal cancer |
| ERK | extracellular signal-regulated kinase |
| HCC | hepatocellular carcinoma |
| HGD | high grade dysplasia |
| IBD | inflammatory bowel disease |
| IGF | insulin growth factors |
| IL | interleukin |
| IR | insulin resistance |
| LT | liver transplant |
| MAMPs | microorganism-associated molecular patterns |
| MAPK | mitogen-activated protein kinase |
| MetS | metabolic syndrome |
| mTOR | mammalian target of rapamycin |
| NAFL | non-alcoholic fatty liver |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NF-kB | nuclear factor-κ B |
| OR | odds ratio |
| PGE2 | Prostaglandin E2 |
| RR | relative risk |
| SIR | standardized incidence ratio |
| STAT3 | signal transducer and activator of transcription |
| TNF-α | tumor necrosis factor α |
| US | ultrasound |
| JAK2 | Janus kinase-2 |
References
- Armstrong, M.J.; Houlihan, D.D.; Bentham, L.; Shaw, J.C.; Cramb, R.; Olliff, S.; Gill, P.S.; Neuberger, J.M.; Lilford, R.J.; Newsome, P.N. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J. Hepatol. 2012, 56, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 2015. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, N.; Bai, C.; Fang, Y.; Srishord, M.; McCullough, A.; Gramlich, T.; Younossi, Z.M. Long-term follow-up of patients with nonalcoholic fatty liver. Clin. Gastroenterol. Hepatol. 2009, 7, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Long-term mortality in nonalcoholic fatty liver disease: Is liver histology of any prognostic significance? Hepatology 2010, 51, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Diehl, A.M. NAFLD and extrahepatic cancers: Have a look at the colon. Gut 2011, 60, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.; Marengo, A.; Mezzabotta, L.; Bugianesi, E. Systemic complications of nonalcoholic fatty liver disease: When the liver is not an innocent bystander. Semin. Liver Dis. 2015, 35, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G. Viewpoints on the way to a consensus session: Where does insulin resistance start? The liver. Diabetes Care 2009, 32, S164–S167. [Google Scholar] [CrossRef] [PubMed]
- Scalera, A.; Tarantino, G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J. Gastroenterol. 2014, 20, 9217–9228. [Google Scholar] [PubMed]
- Hwang, S.T.; Cho, Y.K.; Park, J.H.; Kim, H.J.; Park, D.I.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Won, K.H.; Jin, W. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J. Gastroenterol. Hepatol. 2010, 25, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Lim, Y.-S.; Park, H.S. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: A retrospective cohort study. J. Gastroenterol. Hepatol. 2012, 27, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Wong, G.L.-H.; Tsang, S.W.-C.; Fan, T.; Chu, W.C.; Woo, J.; Chan, A.W.; Choi, P.C.; Chim, A.M.; Lau, J.Y.; et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011, 60, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Stadlmayr, A.; Aigner, E.; Steger, B.; Scharinger, L.; Lederer, D.; Mayr, A.; Strasser, M.; Brunner, E.; Heuberger, A.; Hohla, F.; et al. Nonalcoholic fatty liver disease: An independent risk factor for colorectal neoplasia. J. Intern. Med. 2011, 270, 41–49. [Google Scholar]
- Huang, K.-W.; Leu, H.-B.; Wang, Y.-J.; Luo, J.C.; Lin, H.C.; Lee, F.Y.; Chan, W.L.; Lin, J.K.; Chang, F.Y. Patients with nonalcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy. Colorectal. Dis. 2013, 15, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.T.; Mellemkjaer, L.; Jepsen, P.; Thulstrup, A.M.; Baron, J.; Olsen, J.H.; Vilstrup, H. Risk of cancer in patients hospitalized with fatty liver: A Danish cohort study. J. Clin. Gastroenterol. 2003, 36, 356–359. [Google Scholar]
- Touzin, N.T.; Bush, K.N.V.; Williams, C.D.; Harrison, S.A. Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2011, 4, 169–176. [Google Scholar] [CrossRef]
- Basyigit, S.; Uzman, M.; Kefeli, A.; Sapmaz, F.P.; Yeniova, A.O.; Nazligul, Y.; Asiltürk, Z. Absence of non-alcoholic fatty liver disease in the presence of insulin resistance is a strong predictor for colorectal carcinoma. Int. J. Clin. Exp. Med. 2015, 8, 18601–18610. [Google Scholar] [PubMed]
- Bhatt, B.D.; Lukose, T.; Siegel, A.B.; Brown, R.S.; Verna, E.C. Increased risk of colorectal polyps in patients with non-alcoholic fatty liver disease undergoing liver transplant evaluation. J. Gastrointest. Oncol. 2015, 6, 459–468. [Google Scholar] [PubMed]
- Wong, M.C.S.; Ching, J.Y.L.; Chan, V.C.W.; Lam, T.Y.; Luk, A.K.; Wong, S.H.; Ng, S.C.; Wong, V.W.; Ng, S.S.; Wu, J.C.; et al. Screening strategies for colorectal cancer among patients with nonalcoholic fatty liver disease and family history. Int. J. Cancer 2015. [Google Scholar] [CrossRef]
- Lin, X.F.; Shi, K.Q.; You, J.; Liu, W.Y.; Luo, Y.W.; Wu, F.L.; Chen, Y.P.; Wong, D.K.; Yuen, M.F.; Zheng, M.H. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: A large study. Mol. Biol. Rep. 2014, 41, 2989–2997. [Google Scholar] [PubMed]
- Bilici, A.; Ozguroglu, M.; Mihmanlı, İ.; Turna, H.; Adaletli, İ. A case—Control study of non-alcoholic fatty liver disease in breast cancer. Med. Oncol. 2007, 24, 367–371. [Google Scholar] [CrossRef]
- Vongsuvanh, R.; George, J.; Qiao, L.; van der Poorten, D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett. 2013, 330, 1–10. [Google Scholar]
- Moore, L.L.; Bradlee, M.L.; Singer, M.R.; Splansky, G.L.; Proctor, M.H.; Ellison, R.C.; Kreger, B.E. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 559–567. [Google Scholar]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann. Intern. Med. 1995, 122, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Schoen, R.E.; Tangen, C.M.; Kuller, L.H.; Burke, G.L.; Cushman, M.; Tracy, R.P.; Dobs, A.; Savage, P.J. Increased blood glucose and insulin, body size, and incident colorectal cancer. J. Natl. Cancer Inst. 1999, 91, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Lahmann, P.H.; Boeing, H.; Friedenreich, C.; Norat, T.; Tjønneland, A.; Halkjaer, J.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2006, 98, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Beddy, P.; Howard, J.; McMahon, C.; Knox, M.; de Blacam, C.; Ravi, N.; Reynolds, J.V.; Keogan, M.T. Association of visceral adiposity with oesophageal and junctional adenocarcinomas. Br. J. Surg. 2010, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Kubo, A.; Levin, T.R.; Block, G.; Habel, L.; Zhao, W.; Leighton, P.; Quesenberry, C.; Rumore, G.J.; Buffler, P.A. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology 2007, 133, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, Z.R.; Farrow, D.C.; Bronner, M.P.; Rosen, S.N.; Vaughan, T.L. Central adiposity and risk of Barrett’s esophagus. Gastroenterology 2007, 133, 403–411. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kvapil, P.; Hacken-Bitar, J.; Kramer, J.R. Abdominal obesity and the risk of Barrett’s esophagus. Am. J. Gastroenterol. 2005, 100, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.N.; Murad, M.H.; Buttar, N.S.; El-Serag, H.B.; Katzka, D.A.; Iyer, P.G. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Schulze, M.B.; Pischon, T.; Dietrich, T.; Molina, E.; Chirlaque, M.D.; Barricarte, A.; Amiano, P.; Quirós, J.R.; Tumino, R.; et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Greenwood, D.C.; Chan, D.S.; Vieira, R.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Cade, J.E.; Burley, V.J.; Norat, T. Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non-linear dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Vona-Davis, L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors 2013, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Ricci, C.; Behrens, G.; Leitzmann, M.F. Adiposity and risk of thyroid cancer: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- McGrowder, D.A.; Jackson, L.A.; Crawford, T.V. Prostate cancer and metabolic syndrome: Is there a link? Asian Pac. J. Cancer Prev. 2012, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Merry, A.; Schouten, L.; Goldbohm, R.; van Den Brandt, P. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardias: A prospective cohort study. Gut 2007, 56, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.; Ergun, G.; Pandolfino, J.; Fitzgerald, S.; Tran, T.; Kramer, J. Obesity increases oesophageal acid exposure. Gut 2007, 56, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Cook, M.; Shaheen, N.; Vaughan, T.; Whiteman, D.; Murray, L.; Corley, D.A. Sexspecific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: A pooled analysis from the international BEACON consortium. Gut 2013, 62, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.; Hashmi, A.; Garcia, J.; Richardson, P.; Alsarraj, A.; Fitzgerald, S.; Vela, M.; Shaib, Y.; Abraham, N.S.; Velez, M.; et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: A case-control study. Gut 2014, 63, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Splenser, A.; Kramer, J.; Alsarraj, A.; Fitzgerald, S.; Ramsey, D.; El-Serag, H.B. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett’s esophagus: A case-control study. Clin. Gastroenterol. Hepatol. 2014, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Uzel, M.; Sahiner, Z.; Filik, L. Non-alcoholic fatty liver disease, metabolic syndrome and gastric cancer: Single center experience. J. BUON 2015, 20, 662. [Google Scholar] [PubMed]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Algaba, F.; Patard, J.J.; Khoo, V.; Eisen, T.; Horwich, A. Renal cell carcinoma: ESMO clinical practice guidelines. Ann. Oncol. 2014, 25, iii49–iii56. [Google Scholar] [CrossRef] [PubMed]
- Stocks, T.; Bjørge, T.; Ulmer, H.; Manjer, J.; Häggström, C.; Nagel, G.; Engeland, A.; Johansen, D.; Hallmans, G.; Selmer, R.; et al. Metabolic risk score and cancer risk: Pooled analysis of seven cohorts. Int. J. Epidemiol. 2015, 44, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.K.; Zhang, H.L.; Yao, X.D.; Zhang, S.L.; Dai, B.; Shen, Y.J.; Liu, X.H.; Zhou, L.P.; Ye, D.W. Visceral obesity and risk of high grade disease in clinical T1A renal cell carcinoma. J. Urol. 2013, 189, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Ito, K.; Sumitomo, M.; Kimura, F.; Asano, T.; Hayakawa, M. Decreased serum adiponectin levels in patients with metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 2008, 38, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Smith, G.D.; Ebrahim, S. Hyperinsulinaemia and increased risk of breast cancer: Findings from the British Women’s Heart and Health Study. Cancer Causes Control 2004, 15, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Bosetti, C.; Talamini, R.; Levi, F.; Montella, M.; Giacosa, A.; Negri, E.; La Vecchia, C. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann. Oncol. 2011, 22, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, C.; Berrino, F.; Abagnato, C.A.; Muti, P.; Panico, S.; Crosignani, P.; Krogh, V. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: A nested case-control study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Berrino, F.; Villarini, A.; Traina, A.; Bonanni, B.; Panico, S.; Mano, M.P.; Mercandino, A.; Galasso, R.; Barbero, M.; Simeoni, M.; et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res. Treat. 2014, 147, 159–165. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, R.J.; English, D.R. Body size and composition and prostate cancer risk: Systematic review and meta-regression analysis. Cancer Causes Control 2006, 17, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.M.; Lee, J.H.; Yoon, J.H.; Kwak, C.; Lee, Y.J.; Cho, Y.Y.; Lee, Y.B.; Yu, S.J.; Kim, Y.J.; Kim, H.H.; et al. Nonalcoholic fatty liver disease is a negative risk factor for prostate cancer recurrence. Endocr. Relat. Cancer 2014, 21, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Arase, Y.; Kobayashi, M.; Suzuki, F.; Suzuki, Y.; Kawamura, Y.; Akuta, N.; Imai, N.; Kobayashi, M.; Sezaki, H.; Matsumoto, N.; et al. Difference in malignancies of chronic liver disease due to non-alcoholic fatty liver disease or hepatitis C in Japanese elderly patients. Hepatol. Res. 2012, 42, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor α in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Yoshimoto, S.; Hara, E. Obesity and cancer: A gut microbial connection. Cancer Res. 2014, 74, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y. What is obesity doing to your gut? Malays. J. Med. Sci. 2015, 22, 1–3. [Google Scholar] [PubMed]
- Moran, C.P.; Shanahan, F. Gut microbiota and obesity: Role in aetiology and potential therapeutic target. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Mehal, W.Z. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Spruss, A.; Kanuri, G.; Wagnerberger, S.; Haub, S.; Bischoff, S.C.; Bergheim, I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009, 50, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.A.; Slingerland, J.M. Cytokines, obesity, and cancer: New insights on mechanisms linking obesity to cancer risk and progression. Annu. Rev. Med. 2013, 64, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.M.; Hodge, A.; Farrell, G.C.; Kench, J.G.; Kriketos, A.; George, J. Beyond insulin resistance in NASH: TNF-α or adiponectin? Hepatology 2004, 40, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Nutrition, insulin, insulin-like growth factors and cancer. Horm. Metab. Res. 2003, 35, 694–704. [Google Scholar] [PubMed]
- Pérez-Hernández, A.I.; Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front. Endocrinol. 2014, 5, 65. [Google Scholar] [CrossRef]
- Van Kruijsdijk, R.C.M.; van der Wall, E.; Visseren, F.L.J. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 2000, 183, 1–9. [Google Scholar] [CrossRef]
- Chan, J.M.; Stampfer, M.J.; Giovannucci, E.; Gann, P.H.; Ma, J.; Wilkinson, P.; Hennekens, C.H.; Pollak, M. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science 1998, 279, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Pollak, M.N.; Platz, E.A.; Willett, W.C.; Stampfer, M.J.; Majeed, N.; Colditz, G.A.; Speizer, F.E.; Hankinson, S.E. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol. Biomark. Prev. 2000, 9, 345–349. [Google Scholar]
- Yu, H.; Spitz, M.R.; Mistry, J.; Gu, J.; Hong, W.K.; Wu, X. Plasma levels of insulin-like growth factor-I and lung cancer risk: A case-control analysis. J. Natl. Cancer Inst. 1999, 91, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, S.E.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Michaud, D.S.; Deroo, B.; Rosner, B.; Speizer, F.E.; Pollak, M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998, 351, 1393–1396. [Google Scholar] [CrossRef]
- Donohoe, C.L.; O’Farrell, N.J.; Doyle, S.L.; Reynolds, J.V. The role of obesity in gastrointestinal cancer: Evidence and opinion. Ther. Adv. Gastroenterol. 2014, 7, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; Donohoe, C.L.; Finn, S.P.; Howard, J.M.; Lithander, F.E.; Reynolds, J.V.; Pidgeon, G.P.; Lysaght, J. IGF-1 and its receptor in esophageal cancer: Association with adenocarcinoma and visceral obesity. Am. J. Gastroenterol. 2012, 107, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Siahpush, S.H.; Vaughan, T.L.; Lampe, J.N.; Freeman, R.; Lewis, S.; Odze, R.D.; Blount, P.L.; Ayub, K.; Rabinovitch, P.S.; Reid, B.J.; et al. Longitudinal study of insulin-like growth factor, insulin-like growth factor binding protein-3, and their polymorphisms: Risk of neoplastic progression in Barrett’s esophagus. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Hosono, K.; Uchiyama, T.; Sakai, E.; Sugiyama, M.; Takahashi, H.; Nakajima, N.; Wada, K.; Takeda, K.; Nakagama, H.; et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 2011, 60, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, T.; Schwartz, B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int. J. Cancer 2008, 123, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.F.; Wang, T.; Gunter, M.J.; Strickler, H.D.; Cushman, M.; Kaplan, R.C.; Wassertheil-Smoller, S.; Xue, X.; Rajpathak, S.N.; Chlebowski, R.T.; et al. Adipokines linking obesity with colorectal cancer risk in postmenopausal women. Cancer Res. 2012, 72, 3029–3037. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.H.; Dahlkemper, A.; Kao, J.Y.; Zhang, M.; Morgenstern, H.; McMahon, L.; Inadomi, J.M. A pilot study of the association of low plasma adiponectin and Barrett’s esophagus. Am. J. Gastroenterol. 2008, 103, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.H.; Morgenstern, H.; McConell, D.; Scheiman, J.M.; Schoenfeld, P.; Appelman, H.; McMahon, L.F., Jr.; Kao, J.Y.; Metko, V.; Zhang, M.; et al. Associations of diabetes mellitus, insulin, leptin, and ghrelin with gastroesophageal reflux and Barrett’s esophagus. Gastroenterology 2013, 145, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Healy, L.A.; Power, D.G.; Byrne, M.; Murphy, S.; Byrne, P.J.; Kelleher, D.; Reynolds, J.V. Barrett esophagus: Prevalence of central adiposity, metabolic syndrome, and a proinflammatory state. Ann. Surg. 2008, 247, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Chandar, A.K.; Devanna, S.; Lu, C.; Singh, S.; Greer, K.; Chak, A.; Iyer, P.G. Association of serum levels of adipokines and insulin with risk of barrett’s esophagus: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 2241–2255. [Google Scholar] [CrossRef] [PubMed]
- Francois, F.; Roper, J.; Goodman, A.J.; Pei, Z.; Ghumman, M.; Mourad, M.; de Perez, A.Z.; Perez-Perez, G.I.; Tseng, C.; Blaser, M.J. The association of gastric leptin with oesophageal inflammation and metaplasia. Gut 2008, 57, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kendall, B.J.; Macdonald, G.A.; Hayward, N.K.; Prins, J.B.; Brown, I.; Walker, N.; Pandeya, N.; Green, A.C.; Webb, P.M.; Whiteman, D.C.; et al. Leptin and the risk of Barrett’s oesophagus. Gut 2008, 57, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Delort, L.; Rossary, A.; Farges, M.-C.; Vasson, M.-P.; Caldefie-Chézet, F. Leptin, adipocytes and breast cancer: Focus on inflammation and anti-tumor immunity. Life Sci. 2015, 140, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C.; Mantovani, G. Adipose tissue as target organ in the treatment of hormone-dependent breast cancer: New therapeutic perspectives. Obes. Rev. 2009, 10, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Caldefie-Chézet, F.; Dubois, V.; Delort, L.; Rossary, A.; Vasson, M.-P. Leptin: Involvement in the pathophysiology of breast cancer. Ann. Endocrinol. 2013, 74, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Filková, M.; Haluzík, M.; Gay, S.; Senolt, L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009, 133, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karapanagiotou, E.M.; Tsochatzis, E.A.; Dilana, K.D.; Tourkantonis, I.; Gratsias, I.; Syrigos, K.N. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC). Lung Cancer 2008, 61, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Tiaka, E.K.; Manolakis, A.C.; Kapsoritakis, A.N.; Potamianos, S.P. The implication of adiponectin and resistin in gastrointestinal diseases. Cytokine Growth Factor Rev. 2011, 22, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Alonso-Iglesias, E. Resistin: Insulin resistance to malignancy. Clin. Chim. Acta 2015, 438, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hursting, S.D.; Dunlap, S.M. Obesity, metabolic dysregulation, and cancer: A growing concern and an inflammatory (and microenvironmental) issue. Ann. N. Y. Acad. Sci. 2012, 1271, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, B.; Datta, J.; Teknos, T.N.; Kumar, P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol. Cancer Res. 2011, 9, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.S.; Stamp, G.W.; Foulkes, W.D.; Eccles, D.; Balkwill, F.R. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J. Clin. Investig. 1993, 91, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Ferrajoli, A.; Keating, M.J.; Manshouri, T.; Giles, F.J.; Dey, A.; Estrov, Z.; Koller, C.A.; Kurzrock, R.; Thomas, D.A.; Faderl, S.; et al. The clinical significance of tumor necrosis factor-α plasma level in patients having chronic lymphocytic leukemia. Blood 2002, 100, 1215–1219. [Google Scholar] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Brooks, R.S.; Ciappio, E.D.; Kim, S.J.; Crott, J.W.; Bennett, G.; Greenberg, A.S.; Mason, J.B. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: A mechanism for obesity-associated colorectal cancer. J. Nutr. Biochem. 2012, 23, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.S.; Talpaz, M.; Kurzrock, R. Autocrine interleukin-6 production in renal cell carcinoma: Evidence for the involvement of p53. Cancer Res. 2002, 62, 932–940. [Google Scholar] [PubMed]
- Kai, H.; Kitadai, Y.; Kodama, M.; Cho, S.; Kuroda, T.; Ito, M.; Tanaka, S.; Ohmoto, Y.; Chayama, K. Involvement of proinflammatory cytokines IL-1β and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005, 25, 709–713. [Google Scholar] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Chang, Y.-F. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J. Surg. Oncol. 2003, 83, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Type of Study | Population Enrolled | Exclusion Criteria | NAFLD Diagnosis | Prevalence of Colorectal Lesions in Patients with NAFLD vs. Patients without NAFLD |
|---|---|---|---|---|---|---|
| Bhatt BD et al. [23] (2015) | USA | Retrospective | 591 pts who completed LT evaluation (68 NAFLD vs. 523 non-NAFLD) | <50 years old at LT; IBD; history of multiple/recurrent adenomas; family history of CRC; known cancer-predisposing gene alteration; history of solid organ transplant; HIV pts; personal history of cancer | Biopsy + clinical criteria | Polyps prevalence: 59% vs. 40%; p < 0.003. OR (Odds Ratio) 2.16; p = 0.003 Adenomatous polyps prevalence: 32% vs. 21%; p = 0.04. OR 1.95, p = 0.02 |
| Basyigit S et al. [22] (2015) | Turkey | Prospective observational | 127 consecutive pts who underwent colonoscopy | Other causes of hepatic disease; incomplete colonoscopy; IBD; active gastrointestinal bleeding; history of colorectal surgery; history of CRC; hereditary cancer syndrome | US | Adenomas prevalence: 20% vs. 25.8%. OR 1 CRC prevalence: 4.6% vs. 24.2%. OR 1 |
| Lin XF et al. [25] (2014) | China | Retrospective and consecutive cohort study | 2315 community subjects who underwent a routine colonoscopy (263 NAFLD vs. 2052 non-NAFLD) | History of CRC, adenoma and polyp; history of other extraintestinal malignancies; contraindications to colonoscopy | US | Total colorectal lesions prevalence: 90.0% vs. 93.3% Adenomatous polyps prevalence: 44.5% vs. 55.7% CRC prevalence: 29.3% vs. 18%; p = 0.001. OR 1.868; 95% CI 1.360–2.567; p < 0.05 |
| Wong VW-S et al. [17] (2012) | China | Cross-sectional | 380 community pts + consecutive pts with biopsy proven NAFLD (in total 199 NAFLD vs. 181-non-NAFLD) | Other causes of hepatic disease; history of CRC or polyps; IBD; bowel symptoms including per rectal bleeding and altered bowel habit; prior CRC screening; contraindications to colonoscopy | Proton-magnetic resonance spectroscopy or liver biopsy | Total polyps prevalence: 52.8% vs. 38.7%; p = 0.057 Adenomatous polyps prevalence: 34.7% vs. 21.5%; p = 0.043. OR 1.61; 95% CI 0.9–2.9; p = 0.11 Villous polyps prevalence: 6% vs. 0.6%; p = 0.042 High grade dysplasia polyps prevalence: 18.1% vs. 5%; p = 0.002 Advance neoplasm prevalence: 18.6% vs. 5.5%; p = 0.005. OR 3.04; 95% CI 1.29–7.2; p = 0.011 CRC 1% vs. 0.6%; p = 0.65 |
| Stadlmayr A et al. [18] (2011) | Austria | Cross-sectional | 1211 consecutive pts who underwent screening colonoscopy (632 NAFLD vs. 597 non-NAFLD) | Incomplete colonoscopy; recent colorectal polypectomy, asymptomatic IBD; extraintestinal malignancies | US + exclusion of other causes of hepatic disease | Total colorectal lesions prevalence: 34% vs. 21.7%; p < 0.001 Tubular adenoma prevalence in men: 34.6% vs. 23.7%; p = 0.006 Rectum adenoma prevalence in men: 11% vs. 3%; p = 0.004 CRC prevalence in men: 1.6% vs. 0.4%; p < 0.001 |
| Lee YI et al. [16] (2011) | South Korea | Retrospective cohort study | 5517 women who underwent life insurance company health examinations (831 NAFLD vs. 4686 non-NAFLD) | Other causes of hepatic disease; history of receiving previous medical insurance benefits | US + exclusion of other causes of hepatic disease | Adenomatous polyps incidence: 628 vs. 185.2/105 person year. RR 1.94; 95% CI 1.11–3.40 CRC incidence: 233.6 vs. 27/105 person year. RR 3.08; 95% CI 1.02–9.34 |
| Touzin NT et al. [21] (2011) | USA | Retrospective cohort study | 233 patients who underwent screening colonoscopies (94 NAFD vs. 139 non-NAFLD) | Not available | US + liver biopsy | Adenomas prevalence: 24.4% vs. 25.1%; p = 1 |
| Huang KW et al. [19] (2012) | Taiwan | Retrospective cohort study | 1522 pts with two consecutive colonoscopies (216 with colorectal adenoma vs. 1306 without colorectal adenoma after negative baseline colonoscopy) | History of colorectal adenoma or CRC; adenomas during baseline colonoscopy; incomplete medical record data; alcohol consumption >20 g/day | US + exclusion of other causes of hepatic disease | NAFLD prevalence: 55.6% vs. 38.8%; p < 0.05. OR = 1.45; 95% CI 1.07–1.98; p = 0.016 |
| Hwang ST et al. [15] (2009) | South Korea | Cross-sectional | 2917 pts who underwent routine colonoscopy (556 with polyps vs. 2361 without polyps) | Incomplete colonoscopies; history of polypectomy; IBD; history of cancer; cancer detected during the study; pts with anticoagulant therapy; other causes of hepatic disease | US | NAFLD prevalence: 41.5% vs. 30.2%; p < 0.001. OR, 1.30; 95% CI 1.02–1.66; p = 0.034 |
| Mechanism | Effects | Extra-Hepatic Site |
|---|---|---|
| Insulin resistance | ||
| ↑ IGF-1 axis | Proliferative and anti-apoptotic effects | Prostate/colorectal/lung/Breast cancers, Barrett’s esophagus, esophageal adenocarcinoma |
| Dysfunctional adipose tissue | ||
| ↓ adiponectin/caspase activation ↓ adiponectin/TNF-α ↑ leptin/MAPK ↑ resistin/NF-κB | Anti-apoptotic effects Proliferation and angiogenesis Invasiveness, motility, lamellipodia formation | Gastrointestinal and extra-intestinal cancer Gastrointestinal and extra-intestinal cancer Colon/breast cancer, Barrett’s esophagus, esophageal adenocarcinoma Breast/gastrointestinal and non-small cell lung cancers |
| Inflammation | ||
| IL-6/JAK/STAT3 and IL-6/MAPK TNF-α/Wnt/β-catenin | Proliferation Angiogenesis, differentiation and metastasis development | Renal/gastric/colorectal cancers Colorectal cancer |
| Gut microbiota | ||
| MAMPs/TLRs Inflammasome-derived IL-18 | Inflammation Anti-apoptotic effects | Colon cancer Colon cancer |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. https://doi.org/10.3390/ijms17050717
Sanna C, Rosso C, Marietti M, Bugianesi E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. International Journal of Molecular Sciences. 2016; 17(5):717. https://doi.org/10.3390/ijms17050717
Chicago/Turabian StyleSanna, Claudia, Chiara Rosso, Milena Marietti, and Elisabetta Bugianesi. 2016. "Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers" International Journal of Molecular Sciences 17, no. 5: 717. https://doi.org/10.3390/ijms17050717