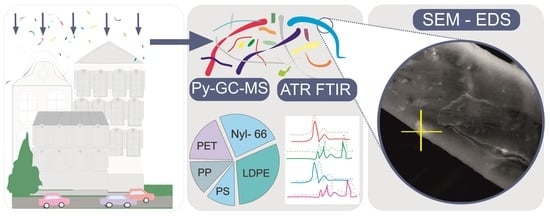
Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.1.1. Study Area
2.1.2. Sampling
2.1.3. Sample Processing
2.1.4. Quality Assurance
2.2. MP Particles’ Identification, Quantification and Characteristics
2.2.1. ATR-FTIR Spectroscopy
2.2.2. Py-GC–MS
2.2.3. Electron Microscope Observation of the MP Surfaces
3. Results and Discussion
3.1. Atmospheric Deposition
3.2. Visual Characteristics of MPs
3.3. Identification of MPs and Analysis of the Change in Their Relative Share over Time
3.3.1. ATR-FTIR Identification
Sample Pre-Treatment with HF
3.3.2. Py-GC–MS Study
A Semi-Quantitative Approach with Regard to the MP Component
3.4. SEM Observations and Microanalysis of the Airborne MP Fibres’ Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Aragaw, T.A. Surgical face masks as a potential source microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Gouin, T.; Roche, N.; Lohmann, R.; Hodges, G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 2011, 45, 1466–1472. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Li, J.; Deng, S.; Zhang, S. Adsorption of three pesticides on polyethylene microplastics in aqueous solutions: Kinetics, isotherms, thermodynamics, and molecular dynamics simulation. Chemosphere 2021, 264, 128556. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro- and nano-plastics and human health. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 343–366. ISBN 978-3-319-16510-3. [Google Scholar]
- Chubarenko, I.; Efimova, I.; Bagaeva, M.; Bagaev, A.; Isachenko, I. On mechanical fragmentation of single-use plastics in the sea swash zone with different types of bottom sediments: Insights from laboratory experiments. Mar. Pollut. Bull. 2020, 150, 110726. [Google Scholar] [CrossRef]
- Ter Halle, A.; Ladirat, L.; Gendre, X.; Goudouneche, D.; Pusineri, C.; Routaboul, C.; Tenailleau, C.; Duployer, B.; Perez, E. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 2016, 50, 5668–5675. [Google Scholar] [CrossRef] [Green Version]
- Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of MPs in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Jiang, J.Q. Occurrence of microplastics and its pollution in the environment: A review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? MPs prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef]
- Gao, X.; Hassan, I.; Peng, Y.; Huo, S.; Ling, L. Behaviors and influencing factors of the heavy metals adsorption onto microplastics: A review. J. Clean. Prod. 2021, 319, 128777. [Google Scholar] [CrossRef]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef]
- Yin, L.; Wen, X.; Huang, D.; Du, C.; Deng, R.; Zhou, Z.; Tao, J.; Li, R.; Zhou, W.; Wang, Z.; et al. Interactions between microplastics/nanoplastics and vascular plants. Environ. Pollut. 2021, 290, 117999. [Google Scholar] [CrossRef]
- Syranidou, E.; Kalogerakis, N. Interactions of microplastics, antibiotics and antibiotic resistant genes within WWTPs. Sci. Total Environ. 2022, 804, 150141. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Singh, L.; Bolan, N.S.; Saha, M. Microplastics as an emerging source of particulate air pollution: A critical review. J. Hazard. Mater. 2021, 418, 126245. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The polymers and their additives in particulate plastics: What makes them hazardous to the fauna? Sci. Total Environ. 2022, 824, 153828. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Vimalkumar, K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front. Endocrinol. 2021, 12, 724978. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Fedeli, U.; Fadda, E.; Milan, G.; Turato, A.; Pavanello, S. Lung cancer risk in workers exposed to poly (vinyl chloride) dust: A nested case-referent study. Occup. Environ. Med. 2003, 60, 423–428. [Google Scholar] [CrossRef]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Sridhar, A.; Kannan, D.; Kapoor, A.; Prabhakar, S. Extraction and detection methods of microplastics in food and marine systems: A critical review. Chemosphere 2022, 286, 131653. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef]
- Zhou, Q.; Tian, C.; Luo, Y. Various forms and deposition fluxes of microplastics identified in the coastal urban atmosphere. Chin. Sci. Bull. 2017, 62, 3902–3909. [Google Scholar] [CrossRef]
- Lim, X.Z. Microplastics are everywhere—But are they harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef]
- Peñalver, R.; Arroyo-Manzanares, N.; López-García, I.; Hernández-Córdoba, M. An overview of microplastics characterization by thermal analysis. Chemosphere 2020, 242, 125170. [Google Scholar] [CrossRef]
- Jung, S.; Cho, S.H.; Kim, K.H.; Kwon, E.E. Progress in quantitative analysis of microplastics in the environment: A review. Chem. Eng. J. 2021, 422, 130154. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Wilczyńska-Michalik, W.; Borek, K.; Michalik, M. Cząstki mikroplastiku w powietrzu atmosferycznym w Krakowie. Aura 2019, 9, 6–10. [Google Scholar] [CrossRef]
- Rataj, M.; Holewa-Rataj, J. Analysis of air quality changes in Małopolska in the years 2012–2020. Nafta-Gaz 2020, 11, 854–863. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Wezel, A.; Caris, I.; Kools, S.A. Release of primary microplastics from consumer products to wastewater in the Netherlands. Environ. Toxicol. Chem. 2016, 35, 1627–1631. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Huang, Y.; Qing, X.; Wang, W.; Han, G.; Wang, J. Mini-review on current studies of airborne microplastics: Analytical methods, occurrence, sources, fate and potential risk to human beings. TrAC Trends Anal. Chem. 2020, 125, 115821. [Google Scholar] [CrossRef]
- Can-Güven, E. Microplastics as emerging atmospheric pollutants: A review and bibliometric analysis. Air Qual. Atmos. Health 2021, 14, 203–215. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Yang, L.; Gao, T.; Zhang, Y. A review of analytical methods and models used in atmospheric microplastic research. Sci. Total Environ. 2022, 828, 154487. [Google Scholar] [CrossRef]
- Massarelli, C.; Campanale, C.; Uricchio, V.F. A Handy Open-Source Application Based on Computer Vision and Machine Learning Algorithms to Count and Classify Microplastics. Water 2021, 13, 2104. [Google Scholar] [CrossRef]
- Urban, M.W. ATR—Attenuated Total Reflectance Spectroscopy of Polymers: Theory and Practice; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar]
- Díaz-Alejo, L.A.; Menchaca, M.; Uruchurtu, J.; Sosa, R.; García-Sánchez, M.A. Effects of the Addition of Ortho- and Para-NH2 Substituted Tetraphenylporphyrins on the Structure of Nylon 66. Int. J. Polym. Sci. 2013, 2013, 323854. [Google Scholar] [CrossRef]
- Dos Santos Pereira, A.P.; Prado da Silva, M.H.; Lima, E.P.; Paula, A.S.; Tommasini, F. Processing and Characterization of PET Composites Reinforced With Geopolymer Concrete Waste. Mater. Res. 2017, 20. [Google Scholar] [CrossRef]
- Noda, I.; Dowrey, A.E.; Haynes, J.L.; Marcott, C. Group Frequency Assignments for Major Infrared Bands Observed in Common Synthetic Polymers. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Maraschin, N. Ethylene polymers, LDPE. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef]
- Matsuo, T. Automotive applications. In Polyesters and Polyamides; Woodhead Publishing: Cambridge, UK, 2008; pp. 525–541. [Google Scholar] [CrossRef]
- Ramli Sulong, N.H.; Mustapa, S.A.S.; Abdul Rashid, M.K. Application of expanded polystyrene (EPS) in buildings and constructions: A review. J. Appl. Polym. Sci. 2019, 136, 47529. [Google Scholar] [CrossRef]
- Mohajerani, A.; Ashdown, M.; Abdihashi, L.; Nazem, M. Expanded polystyrene geofoam in pavement construction. Constr. Build. Mater. 2017, 157, 438–448. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Carta, D.; Cao, G.; D’Angeli, C. Chemical recycling of poly (ethylene terephthalate)(PET) by hydrolysis and glycolysis. Environ. Sci. Pollut. Res. 2003, 10, 390–394. [Google Scholar] [CrossRef]
- Dümichen, E.; Barthel, A.K.; Braun, U.; Bannick, C.G.; Brand, K.; Jekel, M.; Senz, R. Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res. 2015, 85, 451–457. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Sekine, Y.; Otoshi, T. Atmospheric aluminum from human activities. Atmos. Environ. Part B Urban Atmos. 1992, 26, 295–300. [Google Scholar] [CrossRef]
- Wilczyńska-Michalik, W.; Różańska, A.; Bulanda, M.; Chmielarczyk, A.; Pietras, B.; Michalik, M. Physicochemical and microbiological characteristics of urban aerosols in Krakow (Poland) and their potential health impact. Environ. Geochem. Health 2021, 43, 4601–4626. [Google Scholar] [CrossRef]
- Kotowski, T.; Motyka, J.; Knap, W.; Bielewski, J. 17-Year study on the chemical composition of rain, snow and sleet in very dusty air (Krakow, Poland). J. Hydrol. 2020, 582, 124543. [Google Scholar] [CrossRef]
- Conner, T.L.; Williams, R.W. Identification of possible sources of particulate matter in the personal cloud using SEM/EDX. Atmos. Environ. 2004, 38, 5305–5310. [Google Scholar] [CrossRef]





| SAMPLE | D68 | D89 | D99 | D1011 | D1112 | D1201 | D0102 |
|---|---|---|---|---|---|---|---|
| Period of collection | 2 June–9 August 2019 | 9 August–9 September 2019 | 9 September–1 October 2019 | 1 October–5 November 2019 | 5 November–3 December 2019 | 3 December 2019–3 January 2020 | 3 January–2 February 2020 |
| Dry mass of total atmospheric deposition [g] | 0.245 | 0.178 | 0.026 | 0.059 | 0.045 | 0.045 | 0.045 |
| Daily average dry mass of atmospheric deposition [g/day] | 0.0036 | 0.0057 | 0.0012 | 0.0017 | 0.0016 | 0.0016 | 0.0015 |
| ATR-FTIR | X | X | X | X | X | X | X |
| Py-GC–MS | X | X | X | X | X | X | X |
| SEM-EDS | X | ||||||
| Sample preparation procedure | |||||||
| HF pre-treatment | X | ||||||
| Manual concentration | X | X | X | X | X | X | |
| Polymer | Polymer Content [wt.%] | ||||||
|---|---|---|---|---|---|---|---|
| D68 | D89 | D99 | D1011 | D1112 | D1201 | D0102 | |
| PS | 13.6 | – | 0.8 | 1.6 | 0.2 | 22.2 | 16.1 |
| LDPE | 14.7 | 7.0 | 17.7 | 49.4 | 9.6 | 13.8 | 21.9 |
| PUR | – | – | – | – | – | – | |
| Nyl-66 | 66.9 | 93.0 | 56.9 | 47.5 | 89.7 | 64.0 | 34.8 |
| PP | 3.7 | – | 24.6 | 1.0 | 0.2 | – | 27.2 |
| PET | 1.1 | – | – | 0.4 | 0.2 | – | |
| A | Orange MP Fibre Surface | |||||||
|---|---|---|---|---|---|---|---|---|
| wt% | OR 1 | OR 2 | OR 3 | OR 4 | OR 5 | OR 6 | OR 7 | |
| O | 35.35 | 16.74 | n.d. | 33.28 | 36.61 | 47.30 | n.d. | |
| Na | 2.90 | 10.18 | n.d. | 19.72 | 2.17 | n.d. | 5.84 | |
| Mg | 1.30 | n.d. | n.d. | n.d. | 1.65 | 2.19 | n.d. | |
| Al | 12.91 | 0.59 | n.d. | 0.73 | 8.49 | 0.66 | 76.50 | |
| Si | 28.59 | 1.81 | n.d. | 2.27 | 22.56 | 1.21 | n.d. | |
| S | 0.77 | 3.24 | 100.00 | 0.44 | 0.95 | 1.61 | 17.66 | |
| Cl | 4.89 | 39.34 | n.d. | 3.57 | 0.53 | 1.21 | n.d. | |
| K | 5.06 | 21.77 | n.d. | 24.63 | 2.25 | 0.75 | n.d. | |
| Ca | 1.27 | 6.33 | n.d. | 13.09 | 6.05 | 45.08 | n.d. | |
| Cu | n.d | n.d. | n.d. | 2.27 | n.d. | n.d. | n.d. | |
| Ti | 0.31 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Fe | 6.25 | n.d. | n.d. | n.d. | 15.87 | n.d. | n.d. | |
| Zn | 0.42 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Ba | n.d | n.d. | n.d. | n.d. | 0.93 | n.d. | n.d. | |
| P | n.d | n.d. | n.d. | n.d. | 1.91 | n.d. | n.d. | |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| B | Blue MP Fibre Surface | |||||||
| wt% | BLUE 1 | BLUE 2 | BLUE 3 | BLUE 4 | BLUE 5 | BLUE 6 | ||
| O | 46.19 | 47.60 | 43.81 | 14.58 | 18.10 | 42.51 | ||
| Na | 1.66 | 7.14 | 9.74 | 1.52 | n.d | 4.01 | ||
| Mg | n.d | 3.63 | 2.42 | 0.72 | 1.57 | 2.44 | ||
| Al | 1.95 | 1.55 | 1.87 | 7.67 | 1.37 | 8.10 | ||
| Si | 0.96 | 20.40 | 17.18 | 20.63 | 1.01 | 15.15 | ||
| S | 18.24 | 2.67 | 2.86 | 3.57 | 1.72 | 3.87 | ||
| Cl | 2.11 | 8.49 | 10.93 | 12.77 | 0.44 | 7.69 | ||
| K | 1.25 | 5.24 | 6.81 | 11.59 | 0.42 | 5.02 | ||
| Ca | 27.65 | 3.27 | 3.21 | 14.10 | 74.10 | 10.16 | ||
| Cu | n.d | n.d | n.d | n.d | n.d | n.d | ||
| Ti | n.d | n.d | n.d | n.d | n.d | n.d | ||
| Fe | n.d | n.d | 1.17 | 12.87 | n.d | n.d | ||
| Zn | n.d | n.d | n.d | n.d | n.d | n.d | ||
| Ba | n.d | n.d | n.d | n.d | n.d | n.d | ||
| P | n.d | n.d | n.d | n.d | 1.27 | 1.04 | ||
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | ||
| C | Blue MP fibre surface | |||||||
| wt % | BK 1 | BK 2 | BK 3 | BK 4 | BK 5 | BK 6 | BK 7 | BK 8 |
| O | 35.35 | 28.25 | 31.19 | 27.74 | 32.31 | 43.27 | 22.11 | 20.29 |
| Na | 2.90 | 4.50 | n.d | 8.57 | 4.26 | 3.62 | 14.45 | 4.27 |
| Mg | 1.30 | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Al | 12.91 | 16.07 | 38.77 | 16.03 | 1.28 | 0.49 | 1.20 | 13.79 |
| Si | 28.59 | 0.98 | 12.94 | 0.56 | 0.39 | 44.27 | 1.09 | 47.35 |
| S | 0.77 | 5.68 | 1.40 | 3.25 | 0.53 | 1.00 | 5.51 | 0.82 |
| Cl | 4.89 | 25.95 | 7.75 | 24.01 | 3.11 | 4.72 | 31.44 | 11.05 |
| K | 5.06 | 18.56 | 6.22 | 19.83 | 1.34 | 2.21 | 18.54 | 2.43 |
| Ca | 1.27 | n.d | 1.74 | n.d | 56.78 | 0.41 | 4.80 | n.d |
| Cu | n.d | n.d | n.d | n.d | n.d | n.d | 0.87 | n.d |
| Ti | 0.31 | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Fe | 6.25 | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Zn | 0.42 | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Ba | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| P | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| wt% | BK 9 | BK 10 | BK 11 | BK 12 | BK 13 | BK 14 | BK 15 | BK 16 |
| O | 33.79 | n.d | 47.76 | 9.48 | 20.94 | 14.08 | n.d | n.d |
| Na | 5.13 | 1.24 | 4.76 | 8.64 | 2.62 | n.d | 5.30 | n.d |
| Mg | n.d | n.d | n.d | n.d | n.d | n.d | 13.38 | n.d |
| Al | 12.02 | 16.39 | 16.83 | 33.97 | 37.07 | 63.14 | 27.33 | 68.93 |
| Si | 39.83 | 1.34 | 1.50 | 0.71 | 9.96 | n.d | 14.60 | 31.07 |
| S | 0.25 | 29.51 | 10.26 | 1.14 | 2.39 | 0.67 | 3.18 | n.d |
| Cl | 0.31 | 29.20 | 11.23 | 31.17 | 9.50 | 12.99 | 5.18 | n.d |
| K | 8.26 | 22.08 | 5.41 | 14.46 | 7.47 | 5.75 | 3.65 | n.d |
| Ca | n.d | n.d | 2.25 | n.d. | 7.82 | 1.10 | 27.39 | n.d |
| Cu | n.d | n.d | n.d | n.d. | n.d. | 2.27 | n.d | n.d |
| Ti | 0.14 | n.d | n.d | 0.44 | n.d. | n.d. | n.d | n.d |
| Fe | n.d | n.d | n.d | n.d | 1.50 | n.d. | n.d | n.d |
| Zn | n.d | n.d | n.d | n.d | n.d | n.d. | n.d | n.d |
| Ba | 0.28 | n.d | n.d | n.d | n.d | n.d. | n.d | n.d |
| P | n.d | 0.24 | n.d | n.d | 0.73 | n.d. | n.d | n.d |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, K.; Janus, R.; Wądrzyk, M.; Wilczyńska-Michalik, W.; Natkański, P.; Michalik, M. Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study. Int. J. Environ. Res. Public Health 2022, 19, 12252. https://doi.org/10.3390/ijerph191912252
Jarosz K, Janus R, Wądrzyk M, Wilczyńska-Michalik W, Natkański P, Michalik M. Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12252. https://doi.org/10.3390/ijerph191912252
Chicago/Turabian StyleJarosz, Kinga, Rafał Janus, Mariusz Wądrzyk, Wanda Wilczyńska-Michalik, Piotr Natkański, and Marek Michalik. 2022. "Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study" International Journal of Environmental Research and Public Health 19, no. 19: 12252. https://doi.org/10.3390/ijerph191912252