Effect of Epinephrine Administered during Cardiopulmonary Resuscitation on Cerebral Oxygenation after Restoration of Spontaneous Circulation in a Swine Model with a Clinically Relevant Duration of Untreated Cardiac Arrest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Experimental Protocol
2.3. Measurements
2.4. Statistical Analysis
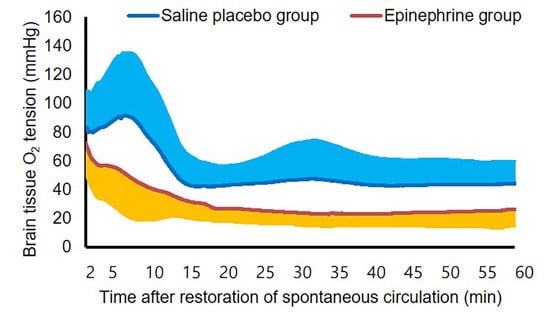
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crile, G.; Dolley, D.H. An Experimental Research into the Resuscitation of Dogs Killed by Anesthetics and Asphyxia. J. Exp. Med. 1906, 8, 713–725. [Google Scholar] [CrossRef]
- Loomba, R.S.; Nijhawan, K.; Aggarwal, S.; Arora, R.R. Increased return of spontaneous circulation at the expense of neurologic outcomes: Is prehospital epinephrine for out-of-hospital cardiac arrest really worth it? J. Crit. Care 2015, 30, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Pocock, H.; et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2018, 379, 711–721. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabanas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Ristagno, G.; Tang, W.; Huang, L.; Fymat, A.; Chang, Y.T.; Sun, S.; Castillo, C.; Weil, M.H. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit. Care Med. 2009, 37, 1408–1415. [Google Scholar] [CrossRef]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit. Care 2014, 21, 1–26. [Google Scholar] [CrossRef]
- Liachenko, S.; Tang, P.; Hamilton, R.L.; Xu, Y. Regional dependence of cerebral reperfusion after circulatory arrest in rats. J. Cereb. Blood Flow Metab. 2001, 21, 1320–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.; Hossmann, K.A. No-reflow after cardiac arrest. Intensive Care Med. 1995, 21, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Manole, M.D.; Kochanek, P.M.; Bayir, H.; Alexander, H.; Dezfulian, C.; Fink, E.L.; Bell, M.J.; Clark, R.S. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental cardiac arrest in rats. Pediatr. Res. 2014, 75, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.G.; Taylor, R.B.; Werman, H.A.; Luu, T.; Spittler, G.; Hamlin, R.L. Effect of standard doses of epinephrine on myocardial oxygen delivery and utilization during cardiopulmonary resuscitation. Crit. Care Med. 1988, 16, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.A.; Otto, C.W.; Kern, K.B.; Hilwig, R.W.; Sanders, A.B.; Henry, C.P.; Ewy, G.A. A randomized, blinded trial of high-dose epinephrine versus standard-dose epinephrine in a swine model of pediatric asphyxial cardiac arrest. Crit. Care Med. 1996, 24, 1695–1700. [Google Scholar] [CrossRef]
- Jeung, K.W.; Ryu, H.H.; Song, K.H.; Lee, B.K.; Lee, H.Y.; Heo, T.; Min, Y.I. Variable effects of high-dose adrenaline relative to standard-dose adrenaline on resuscitation outcomes according to cardiac arrest duration. Resuscitation 2011, 82, 932–936. [Google Scholar] [CrossRef]
- Jung, Y.H.; Ryu, D.H.; Jeung, K.W.; Na, J.Y.; Lee, D.H.; Lee, B.K.; Heo, T.; Min, Y.I. Effect of pralidoxime on coronary perfusion pressure during cardiopulmonary resuscitation in a pig model. Clin. Exp. Emerg. Med. 2019, 6, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Spronk, P.E.; Ince, C.; Gardien, M.J.; Mathura, K.R.; Oudemans-van Straaten, H.M.; Zandstra, D.F. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002, 360, 1395–1396. [Google Scholar] [CrossRef]
- Boerma, E.C.; Mathura, K.R.; van der Voort, P.H.; Spronk, P.E.; Ince, C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: A prospective validation study. Crit. Care 2005, 9, R601–R606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serne, E.H.; Gans, R.O.; ter Maaten, J.C.; Tangelder, G.J.; Donker, A.J.; Stehouwer, C.D. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 2001, 38, 238–242. [Google Scholar] [CrossRef]
- Mekontso-Dessap, A.; Castelain, V.; Anguel, N.; Bahloul, M.; Schauvliege, F.; Richard, C.; Teboul, J.L. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002, 28, 272–277. [Google Scholar] [CrossRef]
- Stocchetti, N.; Zanier, E.R.; Nicolini, R.; Faegersten, E.; Canavesi, K.; Conte, V.; Gattinoni, L. Oxygen and carbon dioxide in the cerebral circulation during progression to brain death. Anesthesiology 2005, 103, 957–961. [Google Scholar] [CrossRef]
- Artru, F.; Dailler, F.; Burel, E.; Bodonian, C.; Grousson, S.; Convert, J.; Renaud, B.; Perret-Liaudet, A. Assessment of jugular blood oxygen and lactate indices for detection of cerebral ischemia and prognosis. J. Neurosurg. Anesthesiol. 2004, 16, 226–231. [Google Scholar] [CrossRef]
- Chieregato, A.; Marchi, M.; Fainardi, E.; Targa, L. Cerebral arterio-venous pCO2 difference, estimated respiratory quotient, and early posttraumatic outcome: Comparison with arterio-venous lactate and oxygen differences. J. Neurosurg. Anesthesiol. 2007, 19, 222–228. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Menon, D.K.; Thiara, S.S.; Cardim, D.; Gupta, A.K.; Hoiland, R.L.; Gooderham, P.; Griesdale, D.E. Brain Hypoxia Secondary to Diffusion Limitation in Hypoxic Ischemic Brain Injury Postcardiac Arrest. Crit. Care Med. 2020, 48, 378–384. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Z.; Zhang, S.; Wu, L.; Li, Y.; Shi, G.; Zhou, J. Mild hypercapnia improves brain tissue oxygen tension but not diffusion limitation in asphyxial cardiac arrest: An experimental study in pigs. BMC Anesthesiol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Flickinger, K.L.; Anderson, M.W.; Koller, A.C.; Sundermann, M.L.; Dezfulian, C.; Okonkwo, D.O.; Shutter, L.A.; Salcido, D.D.; Callaway, C.W.; et al. Effect of neuromonitor-guided titrated care on brain tissue hypoxia after opioid overdose cardiac arrest. Resuscitation 2018, 129, 121–126. [Google Scholar] [CrossRef]
- van den Brink, W.A.; van Santbrink, H.; Steyerberg, E.W.; Avezaat, C.J.; Suazo, J.A.; Hogesteeger, C.; Jansen, W.J.; Kloos, L.M.; Vermeulen, J.; Maas, A.I. Brain oxygen tension in severe head injury. Neurosurgery 2000, 46, 868–876; discussion 876–878. [Google Scholar] [CrossRef]
- Valadka, A.B.; Gopinath, S.P.; Contant, C.F.; Uzura, M.; Robertson, C.S. Relationship of brain tissue PO2 to outcome after severe head injury. Crit. Care Med. 1998, 26, 1576–1581. [Google Scholar] [CrossRef]
- Maloney-Wilensky, E.; Gracias, V.; Itkin, A.; Hoffman, K.; Bloom, S.; Yang, W.; Christian, S.; LeRoux, P.D. Brain tissue oxygen and outcome after severe traumatic brain injury: A systematic review. Crit. Care Med. 2009, 37, 2057–2063. [Google Scholar] [CrossRef]
- Okonkwo, D.O.; Shutter, L.A.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.M.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef]
- Nordmark, J.; Rubertsson, S.; Mortberg, E.; Nilsson, P.; Enblad, P. Intracerebral monitoring in comatose patients treated with hypothermia after a cardiac arrest. Acta Anaesthesiol. Scand. 2009, 53, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, M.S.; Gooderham, P.; Menon, D.K.; Brasher, P.M.A.; Foster, D.; Cardim, D.; Czosnyka, M.; Smielewski, P.; Gupta, A.K.; Ainslie, P.N.; et al. The Burden of Brain Hypoxia and Optimal Mean Arterial Pressure in Patients with Hypoxic Ischemic Brain Injury After Cardiac Arrest. Crit. Care Med. 2019, 47, 960–969. [Google Scholar] [CrossRef]
- Jung, Y.H.; Shamsiev, K.; Mamadjonov, N.; Jeung, K.W.; Lee, H.Y.; Lee, B.K.; Kang, B.S.; Heo, T.; Min, Y.I. Relationship of common hemodynamic and respiratory target parameters with brain tissue oxygen tension in the absence of hypoxemia or hypotension after cardiac arrest: A post-hoc analysis of an experimental study using a pig model. PLoS ONE 2021, 16, e0245931. [Google Scholar] [CrossRef]
- Onetti, Y.; Dantas, A.P.; Perez, B.; Cugota, R.; Chamorro, A.; Planas, A.M.; Vila, E.; Jimenez-Altayo, F. Middle cerebral artery remodeling following transient brain ischemia is linked to early postischemic hyperemia: A target of uric acid treatment. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H862–H874. [Google Scholar] [CrossRef] [PubMed]
- Perez-Asensio, F.J.; de la Rosa, X.; Jimenez-Altayo, F.; Gorina, R.; Martinez, E.; Messeguer, A.; Vila, E.; Chamorro, A.; Planas, A.M. Antioxidant CR-6 protects against reperfusion injury after a transient episode of focal brain ischemia in rats. J. Cereb. Blood Flow Metab. 2010, 30, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.W.; Phan, N.; Ferguson, A.R.; Morabito, D.; Derugin, N.; Stewart, C.L.; Knudson, M.M.; Manley, G.; Rosenthal, G. Brain tissue oxygen tension and its response to physiological manipulations: Influence of distance from injury site in a swine model of traumatic brain injury. J. Neurosurg. 2016, 125, 1217–1228. [Google Scholar] [CrossRef]
- Kiening, K.L.; Unterberg, A.W.; Bardt, T.F.; Schneider, G.H.; Lanksch, W.R. Monitoring of cerebral oxygenation in patients with severe head injuries: Brain tissue PO2 versus jugular vein oxygen saturation. J. Neurosurg. 1996, 85, 751–757. [Google Scholar] [CrossRef]
- Leidorf, A.; Mader, M.M.; Hecker, A.; Heimann, A.; Alessandri, B.; Mayr, P.; Kempski, O.; Wobker, G. Description of the response of a new multi-parametric brain sensor to physiological and pathophysiological challenges in the cortex of juvenile pigs. Turk. Neurosurg. 2014, 24, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.O.; Kanoore Edul, V.S.; Ince, C.; Dubin, A. Comparison of different methods for the calculation of the microvascular flow index. Crit. Care Res. Pract. 2012, 2012, 102483. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Lin, S.; Mohindra, R.; Ramadeen, A.; Toronov, V.; Dorian, P. Study of the Effects of Epinephrine on Cerebral Oxygenation and Metabolism During Cardiac Arrest and Resuscitation by Hyperspectral Near-Infrared Spectroscopy. Crit. Care Med. 2019, 47, e349–e357. [Google Scholar] [CrossRef]
- Artru, A.A.; Nugent, M.; Michenfelder, J.D. Anesthetics affect the cerebral metabolic response to circulatory catecholamines. J. Neurochem. 1981, 36, 1941–1946. [Google Scholar] [CrossRef]
- Menon, D.K.; Coles, J.P.; Gupta, A.K.; Fryer, T.D.; Smielewski, P.; Chatfield, D.A.; Aigbirhio, F.; Skepper, J.N.; Minhas, P.S.; Hutchinson, P.J.; et al. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004, 32, 1384–1390. [Google Scholar] [CrossRef]
- Masamoto, K.; Takizawa, N.; Kobayashi, H.; Oka, K.; Tanishita, K. Dual responses of tissue partial pressure of oxygen after functional stimulation in rat somatosensory cortex. Brain Res. 2003, 979, 104–113. [Google Scholar] [CrossRef]
- Koehler, R.C.; Michael, J.R.; Guerci, A.D.; Chandra, N.; Schleien, C.L.; Dean, J.M.; Rogers, M.C.; Weisfeldt, M.L.; Traystman, R.J. Beneficial effect of epinephrine infusion on cerebral and myocardial blood flows during CPR. Ann. Emerg. Med. 1985, 14, 744–749. [Google Scholar] [CrossRef]
- Michael, J.R.; Guerci, A.D.; Koehler, R.C.; Shi, A.Y.; Tsitlik, J.; Chandra, N.; Niedermeyer, E.; Rogers, M.C.; Traystman, R.J.; Weisfeldt, M.L. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation 1984, 69, 822–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Variable | Placebo Group (n = 12) | Epinephrine Group (n = 11) | p Value 1 |
|---|---|---|---|
| Systolic arterial pressure (mmHg) | 127 ± 14 | 127 ± 17 | 0.988 |
| Diastolic arterial pressure (mmHg) | 87 ± 14 | 84 ± 16 | 0.607 |
| Mean arterial pressure (mmHg) | 104 ± 13 | 103 ± 15 | 0.799 |
| Systolic right atrial pressure (mmHg) | 10 ± 2 | 9 ± 2 | 0.385 |
| Diastolic right atrial pressure (mmHg) | 6 (4–7) | 4 (3–7) | 0.063 |
| Mean right atrial pressure (mmHg) | 8 ± 2 | 7 ± 2 | 0.167 |
| Heart rate (beats/min) | 92 ± 20 | 87 ± 13 | 0.506 |
| End-tidal CO2 (mmHg) | 39 ± 3 | 38 ± 2 | 0.480 |
| Rectal temperature (°C) | 37.2 ± 0.8 | 37.4 ± 0.8 | 0.532 |
| Arterial pH | 7.501 ± 0.040 | 7.523 ± 0.024 | 0.133 |
| PaCO2 (mmHg) | 42.7 ± 3.7 | 40.9 ± 3.0 | 0.223 |
| PaO2 (mmHg) | 172.5 ± 39.0 | 171.8 ± 27.3 | 0.962 |
| SaO2 (%) | 100 (99–100) | 100 (100–100) | 0.247 |
| Arterial lactate (mmol/L) | 0.97 ± 0.24 | 1.01 ± 0.27 | 0.693 |
| Jugular venous pH | 7.424 ± 0.068 | 7.452 ± 0.031 | 0.230 |
| PjvCO2 (mmHg) | 53.3 ± 9.3 | 50.0 ± 5.0 | 0.293 |
| PjvO2 (mmHg) | 48.4 ± 23.0 | 46.5 ± 14.3 | 0.811 |
| SjvO2 (%) | 74 ± 21 | 80 ± 10 | 0.377 |
| Jugular venous lactate (mmol/L) | 1.36 ± 0.51 | 1.38 ± 0.64 | 0.923 |
| Cerebral O2 extraction fraction (%) | 27.7 ± 20.7 | 22.0 ± 10.3 | 0.405 |
| (PjvCO2—PaCO2)/(arterial O2 content—jugular venous O2 content) | 3.33 ± 0.88 | 3.73 ± 1.47 | 0.433 |
| Jugular venous lactate—arterial lactate (mmol/L) | 0.25 (0.12–0.6) | 0.3 (0.2–0.4) | 0.950 |
| Lactate oxygen index | 0.11 (0.08–0.16) | 0.12 (0.09–0.17) | 0.449 |
| PbtO2 at FiO2 0.3 (mmHg) | 31.6 ± 5.6 | 34.1 ± 6.7 | 0.342 |
| PbtO2 at FiO2 1.0 (mmHg) | 74.3 ± 18.6 | 78.5 ± 16.5 | 0.567 |
| Microvascular flow index 2 | 3 | 3 | NA |
| Number of perfused capillaries | 11 ± 3 | 13 ± 3 | 0.190 |
| PjvO2—PbtO2 (mmHg) | 16.8 ± 24.1 | 12.3 ± 16.8 | 0.616 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.Y.; Shamsiev, K.; Mamadjonov, N.; Jung, Y.H.; Jeung, K.W.; Kim, J.W.; Heo, T.; Min, Y.I. Effect of Epinephrine Administered during Cardiopulmonary Resuscitation on Cerebral Oxygenation after Restoration of Spontaneous Circulation in a Swine Model with a Clinically Relevant Duration of Untreated Cardiac Arrest. Int. J. Environ. Res. Public Health 2021, 18, 5896. https://doi.org/10.3390/ijerph18115896
Lee HY, Shamsiev K, Mamadjonov N, Jung YH, Jeung KW, Kim JW, Heo T, Min YI. Effect of Epinephrine Administered during Cardiopulmonary Resuscitation on Cerebral Oxygenation after Restoration of Spontaneous Circulation in a Swine Model with a Clinically Relevant Duration of Untreated Cardiac Arrest. International Journal of Environmental Research and Public Health. 2021; 18(11):5896. https://doi.org/10.3390/ijerph18115896
Chicago/Turabian StyleLee, Hyoung Youn, Kamoljon Shamsiev, Najmiddin Mamadjonov, Yong Hun Jung, Kyung Woon Jeung, Jin Woong Kim, Tag Heo, and Yong Il Min. 2021. "Effect of Epinephrine Administered during Cardiopulmonary Resuscitation on Cerebral Oxygenation after Restoration of Spontaneous Circulation in a Swine Model with a Clinically Relevant Duration of Untreated Cardiac Arrest" International Journal of Environmental Research and Public Health 18, no. 11: 5896. https://doi.org/10.3390/ijerph18115896