A Novel Approach to Derive the Predicted No-Effect Concentration (PNEC) of Benzophenone-3 (BP-3) Using the Species Sensitivity Distribution (SSD) Method: Suggestion of a New PNEC Value for BP-3
Abstract
:1. Introduction
2. Materials and Methods
2.1. BP-3 Toxicity to Aqueous Organisms
2.1.1. BP-3
2.1.2. Aqueous Organisms
2.1.3. Determination of Solubility in the Test Media
2.1.4. Acute Toxicity
2.1.5. Chronic Toxicity
2.1.6. Quantification of BP-3 and Ammonia in the Tested Medium
2.1.7. Statistical Analysis
2.2. Effect Assessment
2.2.1. Toxicity Data Collection
2.2.2. Predicted No-Effect Concentration (PNEC) Derivation
3. Results
3.1. Acute Toxicity
3.2. Chronic Toxicity
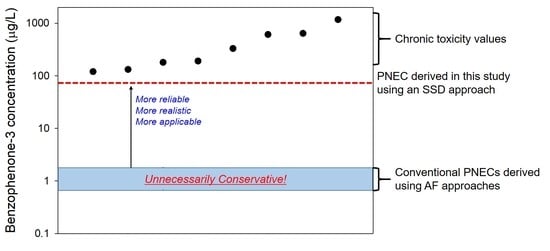
3.3. PNEC Derivation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mao, F.; You, L.; Reinhard, M.; He, Y.; Gin, K.Y.-H. Occurrence and Fate of Benzophenone-Type UV Filters in a Tropical Urban Watershed. Environ. Sci. Technol. 2018, 52, 3960–3967. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are Sunscreens a New Environmental Risk Associated with Coastal Tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Jurado, A.; Gago-Ferrero, P.; Vàzquez-Suñé, E.; Carrera, J.; Pujades, E.; Díaz-Cruz, M.; Barceló, D. Urban Groundwater Contamination by Residues of UV Filters. J. Hazard. Mater. 2014, 271, 141–149. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K. Occurrences, Toxicities, and Ecological Risks of Benzophenone-3, a Common Component of Organic Sunscreen Products: A Mini-Review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The Removal of Pharmaceuticals, Personal Care Products, Endocrine Dis-ruptors and Illicit Drugs during Wastewater Treatment and its Impact on the Quality of Receiving Waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Fent, K. Multiple Hormonal Activities of UV Filters and Comparison of In Vivo and In Vitro Estrogenic Activity of Ethyl-4-Aminobenzoate in Fish. Aquat. Toxicol. 2006, 79, 305–324. [Google Scholar] [CrossRef]
- Vione, D.; Caringella, R.; De Laurentiis, E.; Pazzi, M.; Minero, C. Phototransformation of the Sunlight Filter Benzophenone-3 (2-Hydroxy-4-Methoxybenzophenone) Under Conditions Relevant to Surface Waters. Sci. Total. Environ. 2013, 463, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Ekpeghere, K.I.; Kim, U.J.; Sung-Hee, O.; Kim, H.Y.; Oh, J.E. Distribution and Seasonal Occurrence of UV Filters in Rivers and Wastewater Treatment Plants in Korea. Sci. Total. Environ. 2016, 542, 121–128. [Google Scholar] [CrossRef]
- Nashev, L.G.; Schuster, D.; Laggner, C.; Sodha, S.; Langer, T.; Wolber, G.; Odermatt, A. The UV-Filter Benzophenone-1 Inhibits 17beta-Hydroxysteroid Dehydrogenase Type 3: Virtual Screening as a Strategy to Identify Potential Endocrine Disrupting Chemicals. Biochem. Pharmacol. 2010, 79, 1189–1199. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitamura, S.; Khota, R.; Sugihara, K.; Fujimoto, N.; Ohta, S. Estrogenic and Antiandrogenic Activities of 17 Ben-zophenone Derivatives Used as UV Stabilizers and Sunscreens. Toxicol. Appl. Pharmacol. 2005, 203, 9–17. [Google Scholar] [CrossRef]
- Kawamura, Y.; Ogawa, Y.; Nishimura, T.; Kikuchi, Y.; Nishikawa, J.-I.; Nishihara, T.; Tanamoto, K. Estrogenic Activities of UV Stabilizers Used in Food Contact Plastics and Benzophenone Derivatives Tested by the Yeast Two-Hybrid Assay. J. Health Sci. 2003, 49, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Wang, W.-Q.; Pei, Z.-T.; Ahmad, F.; Xu, R.-R.; Zhang, Y.-M.; Sun, L.-W. Acute Toxicity and Ecological Risk Assessment of Benzophenone-3 (BP-3) and Benzophenone-4 (BP-4) in Ultraviolet (UV)-Filters. Int. J. Environ. Res. Public Health 2017, 14, 1414. [Google Scholar] [CrossRef] [Green Version]
- Sieratowicz, A.; Kaiser, D.; Behr, M.; Oetken, M.; Oehlmann, J. Acute and Chronic Toxicity of Four Frequently Used UV Filter Substances for Desmodesmus Subspicatus and Daphnia Magna. J. Environ. Sci. Health. Part A Toxic Hazard. Subst. Environ. Eng. 2011, 46, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, D.; Kho, Y.; Choi, K. Effects of Benzophenone-3 Exposure on Endocrine Disruption and Reproduction of Japanese Medaka (Oryzias Latipes)—A Two Generation Exposure Study. Aquat. Toxicol. 2014, 155, 244–252. [Google Scholar] [CrossRef]
- Coronado, M.; De Haro, H.; Deng, X.; Rempel, M.A.; Lavado, R.; Schlenk, D. Estrogenic Activity and Reproductive Effects of the UV-Filter Oxybenzone (2-Hydroxy-4-Methoxyphenyl-Methanone) in Fish. Aquat. Toxicol. 2008, 90, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kinnberg, K.L.; Petersen, G.I.; Albrektsen, M.; Minghlani, M.; Awad, S.M.; Holbech, B.F.; Green, J.W.; Bjerregaard, P.; Holbech, H. Endocrine-Disrupting Effect of the Ultraviolet Filter Benzophenone-3 in Zebrafish, Danio Rerio. Environ. Toxicol. Chem. 2015, 34, 2833–2840. [Google Scholar] [CrossRef]
- ECHA. Guidance on information requirements and chemical safety assessment. In Characterisation of Dose [Concentration]-Response for Environment; European Chemicals Agency: Helsinki, Finland, 2008. [Google Scholar]
- ECHA Oxybenzone. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/5515/6/1 (accessed on 20 August 2020).
- Van Straalen, N.M.; Van Leeuwen, C.J. European History of Species Sensitivity Distributions. In Species Sensitivity Distributions in Ecotoxicology; Posthuma, L., Suter, G.W., II, Traas, T.P., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2002. [Google Scholar]
- Warne, M.; Batley, G.; Van Dam, R.; Chapman, J.; Fox, D.; Hickey, C.; Stauber, J.L. Revised Method for Deriving Australian and New Zealand Water Quality Guideline Values for Toxicants; Australian and New Zealand Governments and Australian State and Territory Governments: Canberra, Australia, 2018.
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD Publishing: Paris, France, 2006. [Google Scholar]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Publishing: Paris, France, 2004. [Google Scholar]
- OECD. Test No. 203: Fish, Acute Toxicity Test; OECD Publishing: Paris, France, 1992. [Google Scholar]
- Jung, J.-W.; Kang, J.-S.; Choi, J.; Park, J.-W. Chronic Toxicity of Endocrine Disrupting Chemicals Used in Plastic Products in Korean Resident Species: Implications for Aquatic Ecological Risk Assessment. Ecotoxicol. Environ. Saf. 2020, 192, 110309. [Google Scholar] [CrossRef]
- OECD. Test No. 229: Fish Short Term Reproduction Assay; OECD Publishing: Paris, France, 2011. [Google Scholar]
- Mao, F.; He, Y.; Kushmaro, A.; Gin, K.Y.-H. Effects of Benzophenone-3 on the Green Alga Chlamydomonas Reinhardtii and the Cyanobacterium Microcystis Aeruginosa. Aquat. Toxicol. 2017, 193, 1–8. [Google Scholar] [CrossRef]
- Abbas, H.H. Acute Toxicity of Ammonia to Common Carp Fingerlings (Cyprinus carpio) at Different pH Levels. Pak. J. Biol. Sci. 2006, 9, 2215–2221. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Zha, J.; Xu, Y.; Giesy, J.P.; Richardson, K.L.; Wang, Z. Derivation of Predicted no Effect Concentrations (PNEC) for 2,4,6-Trichlorophenol Based on Chinese Resident Species. Chemosphere 2012, 86, 17–23. [Google Scholar] [CrossRef]
- Hahn, T.; Diamond, J.; Dobson, S.; Howe, P.; Kielhorn, J.; Koennecker, G.; Lee-Steere, C.; Mangelsdorf, I.; Schneider, U.; Sugaya, Y.; et al. Predicted no Effect Concentration Derivation as a Significant Source of Variability in Environmental Hazard Assessments of Chemicals in Aquatic Systems: An International Analysis. Integr. Environ. Assess. Manag. 2013, 10, 30–36. [Google Scholar] [CrossRef]




| Species | C0 (mg/L) | Tested Concentrations (mg/L) | |
|---|---|---|---|
| Acute Tests 1 | Chronic Tests | ||
| Pseudokirchneriella subcapitata | 17.17 ± 0.42 | 0.45, 1.13, 2.83, 7.08, 17.71 | NA 2 |
| Moina macrocopa | 23.55 ± 0.38 | 0.52, 1.00, 2.15, 5.04, 8.25 | 0.012, 0.037, 0.11, 0.33, 1 |
| Cyprinus carpio | 37.40 ± 1.02 | 0.73, 1.18, 2.17, 4.68, 9.35 | 0.0048, 0.024, 0.12, 0.6 |
| Species | Taxonomic Class | Exposure Duration (Days) | Effect | Endpoint | Toxicity Value (μg/L) | Source | Reliability Category |
|---|---|---|---|---|---|---|---|
| Chlamydomonas reinhardtii | green algae | 10 | chl-a reduction | EC20 a | 640 | [26] | 2 |
| Cyprinus carpio | fish | 21 | vitellogenin reduction | NOEC b | 120 | this study | 2 |
| Danio rerio | fish | 60 | skewing of phenotypic sex ratio | NOEC | 191 | [16] | 2 |
| Desmodesmus subspicatus | green algae | 3 | growth inhibition | IC10 c | 560 | [13] | 2 |
| Microcystis aeruginosa | cyanobacteria | 10 | chl-a reduction | EC20 | 1170 | [26] | 2 |
| Moina macrocopa | crustacean | 3 brood | lethality | NOEC | 330 | this study | 2 |
| Oryzias latipes | fish | 21 | reproduction | NOEC | 132 | [15] | 2 |
| Pseudokirchneriella subcapitata | green algae | 3 | growth | NOEC | 180 | this study | 2 |
| Toxicity Data (mg/L) | M. Macrocopa | C. Carpio | |||
|---|---|---|---|---|---|
| Mortality | First Day of Reproduction | Number of Young Per Female | Blood Size | Vtg 1 | |
| LOEC | 1 | >1 | >1 | >1 | 0.6 |
| NOEC | 0.33 | 1 | 1 | 1 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-W.; Kang, J.S.; Choi, J.; Park, J.-W. A Novel Approach to Derive the Predicted No-Effect Concentration (PNEC) of Benzophenone-3 (BP-3) Using the Species Sensitivity Distribution (SSD) Method: Suggestion of a New PNEC Value for BP-3. Int. J. Environ. Res. Public Health 2021, 18, 3650. https://doi.org/10.3390/ijerph18073650
Jung J-W, Kang JS, Choi J, Park J-W. A Novel Approach to Derive the Predicted No-Effect Concentration (PNEC) of Benzophenone-3 (BP-3) Using the Species Sensitivity Distribution (SSD) Method: Suggestion of a New PNEC Value for BP-3. International Journal of Environmental Research and Public Health. 2021; 18(7):3650. https://doi.org/10.3390/ijerph18073650
Chicago/Turabian StyleJung, Jae-Woong, Jae Soon Kang, Jinsoo Choi, and June-Woo Park. 2021. "A Novel Approach to Derive the Predicted No-Effect Concentration (PNEC) of Benzophenone-3 (BP-3) Using the Species Sensitivity Distribution (SSD) Method: Suggestion of a New PNEC Value for BP-3" International Journal of Environmental Research and Public Health 18, no. 7: 3650. https://doi.org/10.3390/ijerph18073650