Modes of Transmission of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) and Factors Influencing on the Airborne Transmission: A Review
Abstract
:1. Introduction
Aim
2. Method
2.1. Design
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Exclusion Criteria
3. Results
Results of the Structured Literature Review
4. Discussion
4.1. Common Symptoms of Coronavirus Disease 2019 (COVID-19)
4.2. Modes of Transmission of SARS-CoV-2
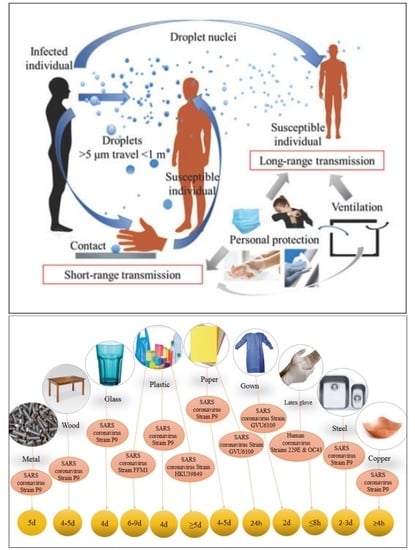
4.2.1. Droplet Transmission
4.2.2. Contact Transmission
4.2.3. Fecal–Oral Transmission
4.2.4. Airborne Transmission
4.3. Concentration and Infectious Dose for Airborne Transmission of SARS-CoV-2
4.4. Influencing Factors on the Airborne Transmission of SARS-CoV-2
4.4.1. Environmental Conditions
4.4.2. Negative Pressure Ventilation (NPV), Displacement Ventilation (DV), Air Conditioning System, Noninvasive Positive Pressure Ventilation (NIPPV), and High-Flow Nasal Cannula (HFNC)
4.5. Protection Approaches for Transmissions of SARS-CoV-2
- (1)
- Isolation of the affected persons, including asymptomatic carriers and travelers from affected countries [122]
- (2)
- Applying travel restrictions from and to infected countries [122]
- (3)
- Avoiding social gatherings and events [122]
- (4)
- Extending knowledge of the public awareness about COVID-19 [122]
- (5)
- (6)
- (7)
- (8)
5. Conclusions
- The airborne transmission of SARS-CoV-2 is an important contributor to fast spreading of the associated disease.
- Droplet transmission occurs from particles >5 µm, which can settle on surfaces under gravitational settling and do not move more than 1 m. Particles <5 µm can stay suspended for an extended period of time (≥2 h) and travel longer distances (up to 8 m) through simple diffusion and convection mechanisms.
- The environmental ambient conditions can affect airborne transmission of SARS-CoV-2 over larger distances.
- The droplets <10 µm in size can be transferred larger distant when the weather is cold and humid.
- The persistence of the virus is remarkable at a low temperature (4 °C), and, by raising the temperature to 70 °C, the virus was no longer detectable after 5 min.
- Although warm weather can slow down the growth rates of SARS-CoV-2, rigid social enforcement and other measures such as the widespread use of face masks are still needed to control the COVID-19 pandemic.
- Coordinated measures among the public and private sectors are needed to control this disease at the national and international level. Joint solutions from different experts, not only in the biomedical sciences, but also in the environment, chemistry, physics, public health, and areas covering transportation, immigration, economic affairs, and education, are required.
- SARS-CoV-2 can also be resuspended from floor surfaces and from protective clothes and shoes of medical workers in indoor environments. Indeed, direct contact with fomites is not the only way for causing viral infection with SARS-CoV-2. As explained above, SARS-CoV-2 is distributed via air. Thus, disinfection of surfaces and protective equipment before removal is needed together with frequent hand washing.
- The various coronaviruses survive on surfaces for up to nine days, and they can be eliminated by disinfection with 62–71% ethanol for 1 min or 0.1% sodium hypochlorite.
- Healthcare workers must be provided with N95, FFP2, or FFP3 masks combined with gowns and goggles.
- Overall, control measures such as using high adequate ventilation, rooms with negative pressure ventilation, practicing social distancing, and wearing N95 and even surgical facemasks are potentially suggested to reduce the SARS-CoV-2 airborne transmission.
5.1. Future Perspective
5.2. Capsule
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman, M.I. An overview of the effect of bioaerosol size in coronavirus disease 2019 transmission. Int. J. Health Plan. Manag. 2020. [Google Scholar] [CrossRef] [PubMed]
- Daniela, D.A.; Gola, M.; Letizia, A.; Marco, D.; Fara, G.M.; Rebecchi, A.; Gaetano, S.; Capolongo, S. COVID-19 and Living Spaces challenge. Well-being and Public Health recommendations for a healthy, safe, and sustainable housing. Acta Biomed. 2020, 91, 61–75. [Google Scholar]
- Li, H.; Wang, Y.; Ji, M.; Pei, F.; Zhao, Q.; Zhou, Y.; Hong, Y.; Han, S.; Wang, J.; Wang, Q. Transmission routes analysis of SARS-CoV-2: A systematic review and case report. Front. Cell Dev. Biol. 2020, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Abkarian, M.; Mendez, S.; Xue, N.; Yang, F.; Stone, H.A. Speech can produce jet-like transport relevant to asymptomatic spreading of virus. Proc. Natl. Acad. Sci. USA 2020, 117, 25237–25245. [Google Scholar] [CrossRef]
- Chong, K.L.; Ng, C.S.; Hori, N.; Yang, R.; Verzicco, R.; Lohse, D. Extended lifetime of respiratory droplets in a turbulent vapour puff and its implications on airborne disease transmission. arXiv 2020, arXiv:2008.01841. [Google Scholar]
- Chen, S.; Yang, J.; Yang, W.; Wang, C.; Bärnighausen, T. COVID-19 control in China during mass population movements at New Year. Lancet 2020. [Google Scholar] [CrossRef] [Green Version]
- Ping, K. Epidemiologic Characteristics of COVID-19 in Guizhou, China. medRxiv 2020. [Google Scholar] [CrossRef]
- WHO. Health Workers Exposure Risk Assessment and Management in the Context of COVID-19 Virus: Interim Guidance, 4 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Xu, C.; Luo, X.; Yu, C.; Cao, S.-J. The 2019-nCoV Epidemic Control Strategies and Future Challenges of Building Healthy Smart Cities; SAGE Publications: London, UK, 2020. [Google Scholar]
- Wu, H.; Huang, J.; Zhang, C.J.; He, Z.; Ming, W.-k. Facemask shortage and the coronavirus disease (COVID-19) outbreak: Reflection on public health measures. medRxiv 2020. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Sánchez-Duque, J.A.; Botero, S.H.; Pérez-Díaz, C.E.; Villamil-Gómez, W.E.; Méndez, C.A.; Verbanaz, S.; Cimerman, S.; Rodriguez-Enciso, H.D.; Escalera-Antezana, J.P. Preparación y control de la enfermedad por coronavirus 2019 (COVID-19) en América Latina. Acta Med. Peru. 2020, 37, 3–7. [Google Scholar] [CrossRef]
- Xiao, Y.; Torok, M.E. Taking the right measures to control COVID-19. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wang, J. A mathematical model for the novel coronavirus epidemic in Wuhan, China. Math. Biosci. Eng. 2020, 17, 2708–2724. [Google Scholar] [CrossRef]
- WHO. Considerations for Quarantine of Individuals in the Context of Containment for Coronavirus Disease (COVID-19): Interim Guidance, 29 February 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- CDC, Centers for Disease Control and Prevention. 2019 Novel Coronavirus (2019-nCoV) Situation Summary. 2020. Available online: https://www.cdc.gov/coronavirus/2019-nCoV/summary.html (accessed on 3 February 2020).
- RCP. Novel Coronavirus 2019|Rubbermaid Commercial Products; ©2020 Rubbermaid Commercial Products LLC 8900 Northpointe Executive Park Drive: Huntersville, NC, USA, 2020. [Google Scholar]
- Bonilla-Aldana, D.K.; Cardona-Trujillo, M.C.; García-Barco, A.; Holguin-Rivera, Y.; Cortes-Bonilla, I.; Bedoya-Arias, H.A.; Patiño-Cadavid, L.J.; Paniz-Mondolfi, A.; Zambrano, L.I.; Dhama, K. MERS-CoV and SARS-CoV Infections in Animals: A Systematic Review and Meta-Analysis of Prevalence Studies. Infez. Med. 2020, 28, 71–83. [Google Scholar] [PubMed]
- Jahanbin, K.; Rahmanian, V. Using twitter and web news mining to predict COVID-19 outbreak. Asian Pac. J. Trop. Med. 2020, 13, 378–380. [Google Scholar]
- Kampf, G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect. Prev. Pract. 2020, 2, 100044. [Google Scholar] [CrossRef]
- WHO. The Covid-19 Risk Communication Package for Healthcare Facilities; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Velraj, R.; Haghighat, F. The contribution of dry indoor built environment on the spread of Coronavirus: Data from various Indian states. Sustain. Cities Soc. 2020, 62, 102371. [Google Scholar]
- Rowan, N.J.; Laffey, J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic–Case study from the Republic of Ireland. Sci. Total Environ. 2020, 725, 138532. [Google Scholar] [CrossRef]
- Stariolo, D.A. COVID-19 in air suspensions. arXiv 2020, arXiv:2004.05699. [Google Scholar]
- Holland, M.; Zaloga, D.J.; Friderici, C.S. COVID-19 Personal Protective Equipment (PPE) for the emergency physician. Vis. J. Emerg. Med. 2020, 19, 100740. [Google Scholar] [CrossRef]
- Guzman, M. Bioaerosol Size Effect in COVID-19 Transmission. 2020. Available online: https://www.preprints.org/manuscript/202004.0093/v1/download (accessed on 7 April 2020). [CrossRef]
- CDC. 2019 Novel Coronavirus: How 2019-nCoV Spreads. Available online: https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html (accessed on 3 February 2020).
- Singla, R.; Mishra, A.; Joshi, R.; Jha, S.; Sharma, A.R.; Upadhyay, S.; Sarma, P.; Prakash, A.; Medhi, B. Human animal interface of SARS-CoV-2 (COVID-19) transmission: A critical appraisal of scientific evidence. Vet. Res. Commun. 2020, 44, 119–130. [Google Scholar] [CrossRef]
- Imai, N.; Cori, A.; Dorigatti, I.; Baguelin, M.; Donnelly, C.A.; Riley, S.; Ferguson, N.M. Report 3: Transmissibility of 2019-nCov; Ref. Source; WHO Collaborating Centre for Infectious Disease Modelling, MRC Centre for Global Infectious Disease Analysis, J-IDEA, Imperial College London: London, UK, 2020; Volume 3, pp. 1–5. [Google Scholar] [CrossRef] [Green Version]
- WHO. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief, 27 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wells, W.F. On air-borne infection: Study ii. droplets and droplet nuclei. Am. J. Epidemiol. 1934, 20, 611–618. [Google Scholar] [CrossRef]
- Wells, W.F.; Stone, W.R. On air-borne infection: Study iii. viability of droplet nuclei infection. Am. J. Epidemiol. 1934, 20, 619–627. [Google Scholar] [CrossRef]
- Guzman, M.I. Bioaerosol Size Effect in COVID-19 Transmission. 2020. Available online: https://www.preprints.org/manuscript/202004.0093/v2/download(accessed on 19 April 2020). [CrossRef] [Green Version]
- Cowling, B.J.; Ip, D.K.; Fang, V.J.; Suntarattiwong, P.; Olsen, S.J.; Levy, J.; Uyeki, T.M.; Leung, G.M.; Peiris, J.M.; Chotpitayasunondh, T. Aerosol transmission is an important mode of influenza A virus spread. Nat. Commun. 2013, 4, 1935. [Google Scholar] [CrossRef]
- Wang, L.-S.; Wang, Y.-R.; Ye, D.-W.; Liu, Q.-Q. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents 2020, 55, 105948. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Li, J.; Yang, J.; Li, J.; Hong, F.; Long, H.; Deng, Q.; Qin, Y.; Jiang, J.; Zhou, X.; et al. SARS-CoV-2 presented in the air of an intensive care unit (ICU). Sustain. Cities Soc. 2020, 102446. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Eykelbosh, A. COVID-19 and Outdoor Safety: Considerations for Use of Outdoor Recreational Spaces; BC Centre for Disease Control|National Collaborating Centre for Environmental Health: Vancouver, BC, Canada, 2020. [Google Scholar]
- Deng, S.-Q.; Peng, H.-J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, S.A.; Gerber, S.I.; Swerdlow, D.L. Middle East respiratory syndrome coronavirus: Update for clinicians. Clin. Infect. Dis. 2015, 60, 1686–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Hilgenfeld, R.; Drag, M. Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Shaikh, A.; Singh, R.; Misra, A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 241–246. [Google Scholar] [CrossRef]
- Liu, F.; Xu, A.; Zhang, Y.; Xuan, W.; Yan, T.; Pan, K.; Yu, W.; Zhang, J. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020, 95, 183–191. [Google Scholar] [CrossRef]
- Hamid, S.; Mir, M.Y.; Rohela, G.K. Novel coronavirus disease (COVID-19): A pandemic (epidemiology, pathogenesis and potential therapeutics). New Microbes New Infect. 2020, 35, 100679. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.-S.; Ye, Z.-W.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. bioRxiv 2020. [Google Scholar] [CrossRef]
- Millán-Oñate, J.; Rodriguez-Morales, A.J.; Camacho-Moreno, G.; Mendoza-Ramírez, H.; Rodríguez-Sabogal, I.A.; Álvarez-Moreno, C. A new emerging zoonotic virus of concern: The 2019 novel Coronavirus (COVID-19). Infectio 2020, 24, 3. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, L.; Ma, J.; Zhou, L. On airborne transmission and control of SARS-Cov-2. Sci. Total Environ. 2020, 731, 139178. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Hama, S.; Omidvarborna, H.; Sharma, A.; Sahani, J.; Abhijith, K.V.; Debele, S.E.; Zavala-Reyes, J.C.; Barwise, Y.; Tiwari, A. Temporary reduction in fine particulate matter due to ‘anthropogenic emissions switch-off’ during COVID-19 lockdown in Indian cities. Sustain. Cities Soc. 2020, 62, 102382. [Google Scholar] [CrossRef]
- Adams, J.G.; Walls, R.M. Supporting the Health Care Workforce During the COVID-19 Global Epidemic. JAMA 2020, 323, 1439–1440. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The, P.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- CDC. Common Human Coronaviruses. 2020. Available online: https://www.cdc.gov/coronavirus/types.html (accessed on 13 February 2020).
- Bonilla-Aldana, D.K.; Villamil-Gómez, W.E.; Rabaan, A.A.; Rodríguez-Morales, A.J. Una nueva zoonosis viral de preocupación global: COVID-19, enfermedad por coronavirus. Iatreia 2019, 33, 107–110. [Google Scholar] [CrossRef]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, evaluation and treatment coronavirus (COVID-19). In Statpearls [Internet]; StatPearls Publishing: Bethesda, MD, USA, 2020. [Google Scholar]
- Encyclopaedia Britannica. Coronavirus—Britannica Online Encyclopedia; Encyclopaedia Britannica, Inc., 2020. Available online: https://www.britannica.com/print/article/138325 (accessed on 13 June 2020).
- Chhikara, B.S.; Rathi, B.; Singh, J.; Poonam, F. Corona virus SARS-CoV-2 disease COVID-19: Infection, prevention and clinical advances of the prospective chemical drug therapeutics. Chem. Biol. Lett. 2020, 7, 63–72. [Google Scholar]
- WHO. Coronavirus. 2020. Available online: https://www.who.int/health-topics/coronavirus (accessed on 3 February 2020).
- Khan, S.; Ali, A.; Siddique, R.; Nabi, G. Novel coronavirus is putting the whole world on alert. J. Hosp. Infect. 2020, 104, 252–253. [Google Scholar] [CrossRef] [Green Version]
- Grimmond, T. Trustworthy Facts on 2019-nCoV and COVID-19 Waste Handling–An Update. 2020. Available online: https://www.danielshealth.com/sites/danielshealth.com/files/PDFs/Other/nCoV-Facts-And-Waste-Handling.pdf (accessed on 13 February 2020).
- Cheng, V.C.; Wong, S.-C.; To, K.K.; Ho, P.; Yuen, K.-Y. Preparedness and proactive infection control measures against the emerging Wuhan coronavirus pneumonia in China. J. Hosp. Infect. 2020, 104, 254–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Dietz, L.; Horve, P.F.; Coil, D.A.; Fretz, M.; Eisen, J.A.; Van Den Wymelenberg, K. 2019 Novel Coronavirus (COVID-19) Pandemic: Built Environment Considerations To Reduce Transmission. Msystems 2020, 5. [Google Scholar] [CrossRef] [Green Version]
- Bourouiba, L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, S.; Zaleski, S.; Soldati, A.; Ahmadi, G.; Bourouiba, L. Host-to-Host Airborne Transmission as a Multiphase Flow Problem for Science-Based Social Distance Guidelines; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Balachandar, S.; Soldati, A. Multiphase flow community must have a role in predicting host-to-host airborne contagion. Int. J. Multiph. Flow 2020, 132, 103440. [Google Scholar] [CrossRef]
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 respirators be reused after disinfection? And for how many times? medRxiv, 2020. [Google Scholar] [CrossRef]
- Gralton, J.; Tovey, E.; McLaws, M.-L.; Rawlinson, W.D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef]
- Nicas, M.; Nazaroff, W.W.; Hubbard, A. Toward understanding the risk of secondary airborne infection: Emission of respirable pathogens. J. Occup. Environ. Hyg. 2005, 2, 143–154. [Google Scholar] [CrossRef]
- Sun, C.; Zhai, Z. The efficacy of social distance and ventilation effectiveness in preventing COVID-19 transmission. Sustain. Cities Soc. 2020, 62, 102390. [Google Scholar] [CrossRef] [PubMed]
- Cook, T. Personal protective equipment during the COVID-19 pandemic—A narrative review. Anaesthesia 2020. [Google Scholar] [CrossRef] [Green Version]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Maroufi, P.; Momen-Heravi, M.; Dao, S.; Köse, S.; Ganbarov, K.; Pagliano, P.; Esposito, S.; Kafil, H.S. Protection and disinfection policies against SARS-CoV-2 (COVID-19). Infez. Med. 2020, 28, 185–191. [Google Scholar]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; La Bruna, A.; Romano, G.L. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R. Early GI Symptoms in COVID-19 May Indicate Fecal Transmission. Gastroenterology 2020. [Google Scholar] [CrossRef]
- Liao, W.; Mui, K.; Cheng, C.; Wong, L.; He, K. Air pressure fluctuations of drainage stacks at a high-rise office building. Indoor Built Environ. 2011, 20, 412–419. [Google Scholar] [CrossRef]
- Dehghani, M.; Sorooshian, A.; Ghorbani, M.; Fazlzadeh, M.; Miri, M.; Badiee, P.; Parvizi, A.; Ansari, M.; Baghani, A.N.; Delikhoon, M. Seasonal variation in culturable bioaerosols in a wastewater treatment plant. Aerosol Air Qual. Res. 2018, 18, 2826–2839. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, M.; Sorooshian, A.; Nazmara, S.; Baghani, A.N.; Delikhoon, M. Concentration and type of bioaerosols before and after conventional disinfection and sterilization procedures inside hospital operating rooms. Ecotoxicol. Environ. Saf. 2018, 164, 277–282. [Google Scholar] [CrossRef]
- Chegini, F.M.; Baghani, A.N.; Hassanvand, M.S.; Sorooshian, A.; Golbaz, S.; Bakhtiari, R.; Ashouri, A.; Joubani, M.N.; Alimohammadi, M. Indoor and outdoor airborne bacterial and fungal air quality in kindergartens: Seasonal distribution, genera, levels, and factors influencing their concentration. Build. Environ. 2020, 175, 106690. [Google Scholar] [CrossRef]
- Baghani, N.A.; Sorooshian, A.; Delikhoon, M.; Nabizadeh, R.; Nazmara, S.; Bakhtiari, R. Pollution characteristics and noncarcinogenic risk assessment of fungal bioaerosol in different processing units of waste paper and cardboard recycling factory. Toxin Rev. 2020, 1–12. [Google Scholar] [CrossRef]
- Hamzavi, S.S.; Amanati, A.; Badiee, P.; Kadivar, M.R.; Jafarian, H.; Ghasemi, F.; Haghpanah, S.; Dehghani, M.; Baghani, A.N. Changing face of Candida colonization pattern in pediatric patients with hematological malignancy during repeated hospitalizations, results of a prospective observational study (2016–2017) in shiraz, Iran. BMC Infect. Dis. 2019, 19, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lednicky, J.A.; Lauzard, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, L. COMMENTARY: COVID-19 Transmission Messages should Hinge on Science-6/12/2020. 2020. Available online: https://www.cidrap.umn.edu/news-perspective/2020/03/commentary-covid-19-transmission-messages-should-hinge-science (accessed on 1 November 2020).
- Eikenberry, S.E.; Mancuso, M.; Iboi, E.; Phan, T.; Eikenberry, K.; Kuang, Y.; Kostelich, E.; Gumel, A.B. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. arXiv 2020, arXiv:2004.03251. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.U. Minimum Sizes of Respiratory Particles Carrying SARS-CoV-2 and the Possibility of Aerosol Generation. Int. J. Environ. Res. Public Health 2020, 17, 6960. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fink, J.B.; Ehrmann, S. High-flow nasal cannula for COVID-19 patients: Low risk of bio-aerosol dispersion. Eur. Respir. J. 2020, 55, 2000892. [Google Scholar] [CrossRef]
- Ufficiale, L.G.; Prima, P.; Generale, S. Decreto del presidente del consiglio dei ministri 10 Aprile 2020. Gazz. Uff. Della Repubb. Ital. 2020, 46, 1–39. [Google Scholar]
- WHO. Responding to Community Spread of COVID-19: Interim Guidance, 7 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Fineberg, H.; Council, N.R. Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic (1 April 2020); The National Academies Press, National Research Council: Washington, DC, USA, 2020. [Google Scholar]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect. Dis. 2019, 19, 101. [Google Scholar] [CrossRef]
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.; Mirchandani, D.; Plante, J.; Aguilar, P.V.; Fernandez, D. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Read, R. A choir decided to go ahead with rehearsal. Now dozens of members have COVID-19 and two are dead. Los Angeles Times, 29 March 2020. Available online: https://www.latimes.com/world-nation/story/2020-03-29/coronavirus-choir-outbreak(accessed on 6 May 2020).
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Gu, J.; Li, K.; Xu, C.; Su, W.; Lai, Z.; Zhou, D.; Yu, C.; Xu, B.; Yang, Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1628–1631. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Surace, G.; Paduano, V.; Fiorillo, M.T.; De Stefano, R.; Laudicella, R.; Baldari, S.; Gaeta, M.; Cicciù, M. SARS-CoV-2 persistence: Data summary up to Q2 2020. Data 2020, 5, 81. [Google Scholar] [CrossRef]
- Guo, Z.-D.; Wang, Z.-Y.; Zhang, S.-F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.-B.; Dong, Y.-Z.; Chi, X.-Y. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26, 1583–1591. [Google Scholar] [CrossRef]
- Geddes, L. Does a high viral load or infectious dose make covid-19 worse. New Scientist. 27 March 2020. Available online: https://www.newscientist.com/article/2238819-does-a-high-viral-load-or-infectious-dose-make-covid-19-worse/ (accessed on 5 April 2020).
- CDC. Public Health Recommendations after Travel-Associated COVID-19 Exposure; 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html (accessed on 4 April 2020).
- Cheval, S.; Mihai Adamescu, C.; Georgiadis, T.; Herrnegger, M.; Piticar, A.; Legates, D.R. Observed and Potential Impacts of the COVID-19 Pandemic on the Environment. Int. J. Environ. Res. Public Health 2020, 17, 4140. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Chong, K.L.; Yang, R.; Li, M.; Verzicco, R.; Lohse, D. Growth of respiratory droplets in cold and humid air. arXiv 2020, arXiv:2011.01515. [Google Scholar]
- Chaudhuri, S.; Basu, S.; Kabi, P.; Unni, V.R.; Saha, A. Modeling ambient temperature and relative humidity sensitivity of respiratory droplets and their role in Covid-19 outbreaks. arXiv 2020, arXiv:2004.10929. [Google Scholar]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.; Morwitzer, M.J.; Creager, H.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.; Schnaubelt, E.; Broadhurst, M.J. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Phoon, P.H.Y.; Jing, F.; Ting, L.X. Response and operating room preparation for the COVID-19 outbreak: A perspective from the National Heart Centre Singapore. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2331–2337. [Google Scholar] [CrossRef]
- Vukkadala, N.; Qian, Z.J.; Holsinger, F.C.; Patel, Z.M.; Rosenthal, E. COVID-19 and the otolaryngologist–preliminary evidence-based review. Laryngoscope 2020, 130, 2537–2543. [Google Scholar] [CrossRef] [Green Version]
- CDC (Centers for Disease Control and Prevention). Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons under Investigation for COVID-19 in Healthcare Settings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html (accessed on 18 May 2020).
- Chiu, P.W.Y.; Ng, S.C.; Inoue, H.; Reddy, D.N.; Hu, E.L.; Cho, J.Y.; Ho, L.K.; Hewett, D.G.; Chiu, H.-M.; Rerknimitr, R. Practice of endoscopy during COVID-19 pandemic: Position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut 2020, 69, 991–996. [Google Scholar] [CrossRef]
- Bhagat, R.K.; Davies Wykes, M.S.; Dalziel, S.B.; Linden, P.F. Effects of ventilation on the indoor spread of COVID-19. J. Fluid Mech. 2020, 903, F1. [Google Scholar] [CrossRef]
- Miller, D.C.; Beamer, P.; Billheimer, D.; Subbian, V.; Sorooshian, A.; Campbell, B.S.; Mosier, J.M. Aerosol Risk with Noninvasive Respiratory Support in Patients with COVID-19. J. Am. Coll. Emerg. Physicians Open 2020. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.; Rozhnova, G.; van Boven, M. Isolation and contact tracing can tip the scale to containment of COVID-19 in populations with social distancing. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Mittal, R.; Ni, R.; Seo, J.-H. The flow physics of COVID-19. J. Fluid Mech. 2020, 894, F2. [Google Scholar] [CrossRef]
- Adlhoch, C.; Baka, A.; Ciotti, M.; Gomes, J.; Kinsman, J.; Leitmeyer, K.; Melidou, A.; Noori, T.; Pharris, A.; Penttinen, P. Considerations Relating to Social Distancing Measures in Response to the COVID-19 Epidemic; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2020. [Google Scholar]
- Cheng, H.-Y.; Jian, S.-W.; Liu, D.-P.; Ng, T.-C.; Huang, W.-T.; Lin, H.-H. High transmissibility of COVID-19 near symptom onset. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- WHO. Management of Ill Travellers at Points of Entry–International Airports, Seaports and Ground Crossings–in the Context of COVID-19 Outbreak: Interim Guidance, 16 February 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Aguirre-Duarte, N. Can people with asymptomatic or pre-symptomatic COVID-19 infect others: A systematic review of primary data. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.-H.; Ni, W.; Wu, Q.; Li, W.-J.; Li, G.-J.; Wang, W.-D.; Tong, J.-N.; Song, X.-F.; Wong, G.W.-K.; Xing, Q.-S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020, 53, 473–480. [Google Scholar] [CrossRef]
- Sultan, S.; Lim, J.K.; Altayar, O.; Davitkov, P.; Feuerstein, J.D.; Siddique, S.M.; Falck-Ytter, Y.; El-Serag, H.B. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology 2020, 159, 739–758.e4. [Google Scholar] [CrossRef]
- Leung, N.H.; Chu, D.K.; Shiu, E.Y.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.; Yen, H.-L.; Li, Y.; Ip, D.K.; Peiris, J.M. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Gilkeson, C.; Noakes, C.; Khan, M. Computational fluid dynamics modelling and optimisation of an upper-room ultraviolet germicidal irradiation system in a naturally ventilated hospital ward. Indoor Built Environ. 2014, 23, 449–466. [Google Scholar] [CrossRef] [Green Version]
- Boyce, J.M. Alcohols as surface disinfectants in healthcare settings. Infect. Control Hosp. Epidemiol. 2018, 39, 323–328. [Google Scholar] [CrossRef] [PubMed]
- CDC. Handwashing: When and How to Wash Your Hands. 2020. Available online: https://www.cdc.gov/handwashing/when-how-handwashing.html (accessed on 7 February 2020).
- Srivastava, N.; Saxena, S.K. Prevention and Control Strategies for SARS-CoV-2 Infection. In Coronavirus Disease 2019 (COVID-19): Epidemiology, Pathogenesis, Diagnosis, and Therapeutics; Saxena, S.K., Ed.; Springer: Singapore, 2020; pp. 127–140. [Google Scholar]
- Aman, F.; Masood, S. How Nutrition can help to fight against COVID-19 Pandemic. Pak. J. Med. Sci. 2020, 36, S121–S123. [Google Scholar] [CrossRef] [PubMed]
- Black, J.R.; Bailey, C.; Przewrocka, J.; Dijkstra, K.K.; Swanton, C. COVID-19: The case for health-care worker screening to prevent hospital transmission. Lancet 2020, 395, 1418–1420. [Google Scholar] [CrossRef]
- WHO. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Skali, H.; Murthy, V.L.; Al-Mallah, M.H.; Bateman, T.M.; Beanlands, R.; Better, N.; Calnon, D.A.; Dilsizian, V.; Gimelli, A.; Pagnanelli, R. Guidance and best practices for nuclear cardiology laboratories during the coronavirus disease 2019 (COVID-19) pandemic: An Information Statement from ASNC and SNMMI. J. Nucl. Cardiol. 2020, 1. [Google Scholar] [CrossRef]
- Poon, L.C.; Yang, H.; Lee, J.C.; Copel, J.A.; Leung, T.Y.; Zhang, Y.; Chen, D.; Prefumo, F. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: Information for healthcare professionals. Ultrasound Obstet. Gynecol. 2020, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delikhoon, M.; Guzman, M.I.; Nabizadeh, R.; Norouzian Baghani, A. Modes of Transmission of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) and Factors Influencing on the Airborne Transmission: A Review. Int. J. Environ. Res. Public Health 2021, 18, 395. https://doi.org/10.3390/ijerph18020395
Delikhoon M, Guzman MI, Nabizadeh R, Norouzian Baghani A. Modes of Transmission of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) and Factors Influencing on the Airborne Transmission: A Review. International Journal of Environmental Research and Public Health. 2021; 18(2):395. https://doi.org/10.3390/ijerph18020395
Chicago/Turabian StyleDelikhoon, Mahdieh, Marcelo I. Guzman, Ramin Nabizadeh, and Abbas Norouzian Baghani. 2021. "Modes of Transmission of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) and Factors Influencing on the Airborne Transmission: A Review" International Journal of Environmental Research and Public Health 18, no. 2: 395. https://doi.org/10.3390/ijerph18020395