Physiological Responses of Earthworm Under Acid Rain Stress
Abstract
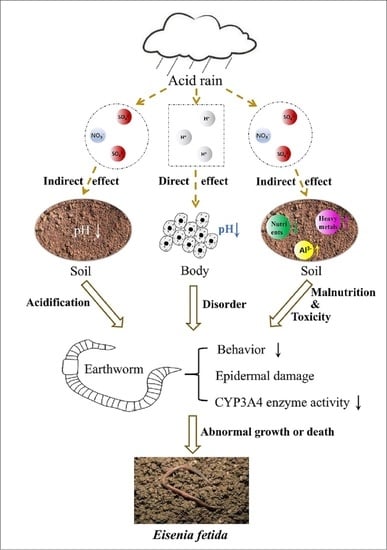
:1. Introduction
2. Materials and Methods
2.1. Experimental Device
2.2. Tested Materials
2.2.1. Preparation of Acid Rain
2.2.2. Tested Earthworm
2.2.3. Tested Soil
2.3. Experimental Design
2.3.1. Exposure Experiments
2.3.2. Measurement of CYP3A4 Enzyme Activity
2.3.3. Scanning of the Electron Microscope of Earthworm Epidermis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Behavior and Mortality of Earthworms
3.2. The Epidermal Damage of Earthworms
3.3. CYP3A4 Enzyme Activity in Earthworms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Y.; Wang, B.; Zhang, R.; Gao, Y.; Zhang, X.; Li, Y.; Zuo, Z. Initial simulated acid rain impacts reactive oxygen species metabolism and photosynthetic abilities in Cinnamonum camphora undergoing high temperature. Ind. Crop. Prod. 2019, 135, 352–361. [Google Scholar] [CrossRef]
- Haradhan, K.M. Acid rain is a local environmental pollution but global concern. Open Sci. J. Anal. Chem. 2018, 35, 47–55. [Google Scholar]
- Wan, Y.S.; Yu, W.M. Analysis on current situation, formation causes and control countermeasures of acid rain pollution in China. J. Anhui Agri. Sci 2010, 38, 19420–19421, 19425. [Google Scholar]
- An, T.C.P.; Gao, W.J. Research on acid rain on the right track. World Sci. 1983, 9, 40–42. [Google Scholar]
- Tripathi, A.K.; Mukesh, G. Biochemical parameters of plants as indicators of air pollution. J. Environ. Biol. 2007, 28, 127–132. [Google Scholar]
- Dwivedi, A.K.; Tripathi, B.D. Pollution tolerance and distribution pattern of plants in surrounding area of coal-fired industries. J. Environ. Biol 2007, 28, 257–263. [Google Scholar] [PubMed]
- Zhang, C.Y.; Yi, X.Q.; Gao, X.Z.; Wang, M.H.; Shao, C.Y.; Lv, Z.D.; Chen, J.J.; Liu, Z.H.; Shen, C.W. Physiological and biochemical responses of tea seedlings (Camellia sinensis) to simulated acid rain conditions. Ecotoxicol. Environ. Saf. 2020, 192, 110315. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; An, W.L.; Liu, X.Y.; Chen, X.X.; Lin, S.Y.; Wang, W.Q. Effects of simulated acid rain on carbon, nitrogen, phosphorus contents and the ecological stoichiometry of rice leaves in Fuzhou rice fields along the river. Acta Ecol. Sin. 2020, 40, 1–11. [Google Scholar]
- Chen, X.X.; An, W.L.; Chen, Y.Y.; Liu, X.Y.; Jing, Q.; Lin, S.Y.; Wang, W.Q. Effects of simulated acid rain on soil carbon pool and soil carbon pool management index of paddy field in Fuzhou plain. J. Soil Water Conserv. 2019, 33, 294–302. [Google Scholar]
- Wei, H.; Ma, R.; Zhang, J.E.; Saleem, M.; Liu, Z.Q.; Shan, X.R.; Yang, J.Y.; Xiang, H.M. Crop-litter type determines the structure and function of litter-decomposing microbial communities under acid rain conditions. Sci. Total Environ. 2020, 713, 136600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Li, D.F.; Zhang, J.E.; Saleem, M.; Zhang, Y.; Ma, R.; He, Y.N.; Yang, J.Y.; Xiang, H.M.; Wei, H. Effect of simulated acid rain on soil CO2, CH4 and N2O emissions and microbial communities in an agricultural soil. Geoderma 2020, 366, 114222. [Google Scholar] [CrossRef]
- Schaefer, M. The soil fauna of a beech forest on limestone: Trophic structure and energy budget. Oecologia 1990, 82, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Visser, S. Role of soil invertebrates in determining the composition of soil microbial communities. Ecol. Interact. Soil 1985, 4, 297–317. [Google Scholar]
- Anderson, J.M. Spatiotemporal effects of invertebrates on soil processes. Biol Fertil Soils 1988, 6, 216–227. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Briones, M.J.I.; Neilson, R.; Schmidt, O.; Spurgeon, D.J.; Creamer, R. A critical review of current methods in earthworm ecology: From individuals to populations. Eur. J. Soil Biol. 2010, 46, 67–73. [Google Scholar] [CrossRef]
- Xu, D.M.; Rao, G.W. Joint effect of co-exposure of Cu and chlorpyrifos on the toxicity of earthworm. Chin. J. Appl. Ecol. 2016, 27, 3029–3034. [Google Scholar]
- Higgins, C.P.; Mcleod, P.B.; MacManus-Spencer, L.A.; Luthy, R. Bioaccumulation of perfluorochemicals in sediments by the aquatic oligochaete lumbriculus variegatus. Environ. Sci. Technol. 2007, 41, 4600–4606. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Q.X.; Wang, Y.Y. Ecotoxicological responses of the earthworm Eisenia fetida exposed to soil contaminated with HHCB. Chemosphere 2011, 83, 1080–1086. [Google Scholar] [CrossRef]
- Qiu, J.P. Earthworms and their application in environment protection Ⅱ. Ecotoxicology of earthworms. J. Shanghai Agric. Coll. 1999, 17, 301–308. (In Chinese) [Google Scholar]
- Fent, K. Ecotoxicological problems associated with contaminatedsites. Toxicol. Lett. 2003, 11, 353–365. [Google Scholar] [CrossRef]
- Chen, T. The status quo of soil heavy metal pollution and its remediation measures in China. Xiangcun Keji 2018, 23, 118–121. [Google Scholar]
- Dai, J.; Liu, T.H. Pollution Characteristics of Ecological Environment of Vegetable Land in Guangzhou. Chin. J. Soil Sci. 1995, 26, 102–104. [Google Scholar]
- An, H.X.; Huang, Z.G. Control measures for heavy metal pollution in acid soils of southern China. China South. Agric. Mach. 2019, 13, 17–22. [Google Scholar]
- Xu, X.H.; Xu, G.L. Spatial distribution of acid rain in acid rain pollution controlled area of China based on GIS. J. Shijiazhuang Univ. 2014, 16, 78–83. [Google Scholar]
- Xu, X.H.; Gao, H.W. Analysis of the distribution and causes of acid rain in Southern China. Sichuan Environ. 2011, 30, 135–139. [Google Scholar]
- Zhang, J.E.; Yu, J.Y.; Ouyang, Y. Activity of Earthworm in latosol under simulated acid rain stress. Bull. Environ. Contam. Toxicol. 2014, 93, 108–111. [Google Scholar] [CrossRef]
- Spatzenegger, M.; Jaeger, W. Clinical importance of hepatic cytochrome-P450 in drug-metabolism. Drug Metab. Rev. 1995, 27, 397–417. [Google Scholar] [CrossRef]
- Sun, J.H. Human Pregnane X Receptor-Mediated Transcriptional Regulation of CYP3A4 by Six Food Non-Nutrients. Master¡¯s Thesis, Zhejiang University, Hangzhou, China, 2004. [Google Scholar]
- Masuyama, H.; Suwaki, N.; Tateishi, Y.; Nakatsukasa, H.; Segawa, T.; Hiramatsu, Y. The pregnane X receptor regulates gene expression in a ligand-and promoter-selective fashion. Mol. Endocrinol. 2005, 19, 1170–1180. [Google Scholar] [CrossRef]
- Takeshita, A.; Koibuchi, N.; Oka, J.; Taguchi, M.; Shishiba, Y.; Ozawa, Y. Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur. J. Endocrinol. 2001, 145, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Han, S.C.; Lv, X.; Li, Z.G.; Fan, Z.W. Effects of simulated acid rain on seed germination and seedling growth of Mikania micrantha H.B.K. Ecol. Environ. Sci. 2010, 19, 679–685. [Google Scholar]
- Dai, Z.M.; Liu, X.M.; Wu, J.J.; Xu, J.M. Impact of simulated acid rain on recalcitrance of two different soils. Environ. Sci. Pollut. Res. 2013, 20, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- Sonderfan, A.J.; Arlotto, M.P.; Dutton, D.R.; Mcmillen, S.; Parkinson, A. Regulation of testosterone hydroxulation by rat-liver microsomal cytochrome-P-450. Arch. Biochem. Biophys. 1987, 255, 27–41. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhu, Y.X.; Gunaratna, C. Rapid and quantitative determination of metabolites from multiple cytochrome P450 probe substrates by gradient liquid chromatography-electrospray ionization-ion trap mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2002, 780, 371–379. [Google Scholar] [CrossRef]
- Kai, J.R.; Song, Y.F.; Cao, X.F.; Yang, X.X.; Yin, Y.C.; Chen, L. Ecotoxic responses of earthworm Eisenia fetida exposed to cypermethrin. J. Agro-Environ. Sci. 2013, 32, 224–231. [Google Scholar]
- Zhou, Y.; Lin, L.; Guo, Y.J.; Liu, Y.; Zhang, L.K.; Liu, D.Y.; Meng, Q.R. Observation of pollen morphologies in nine cultivars of Verbena Linn by Sem. J. Hebei Agric. Univ. 2018, 41, 59–74. [Google Scholar]
- Luo, Q.G.; Chen, X.X.; Zhuang, W.Z. Effects of pesticides on romanomermis wuchangensis a mermithid nematode parasite of mosquito larvae. Chin. J. Vector Biol. Control 1991, 2, 73–76. [Google Scholar]
- Yu, J.Y. Effect of Simulated Acid Rain on Al Activation in Red Soil and Its Ecotoxicity on Earthworms; South China Agricultural University: Guangzhou, China, 2010. [Google Scholar]
- Meng, L.; Zhang, J.E.; Xu, H.Q.; Yu, J.Y.; Qin, Z.; Xie, J.F.; Quan, G.M. Killing effects of compound pollution from the simulated acid rain and its activated Al3+ on small and medium-sized soil animals. Ecol. Environ. Sci. 2011, 20, 1491–1495. [Google Scholar]
- Wang, Y.L. Stress Response of Earthworm to Arsenic Pollution and Its Effects on Soil Physical and Chemical Properties; Shanghai Jiao Tong University: Shanghai, China, 2019. [Google Scholar]
- Hu, C.H.; Zheng, L.Z. Effects of Acidic Sediment on Soil Animals. Hubei Sci Technol 1999, 2, 41. [Google Scholar]
- Li, Y.; Sun, R. Study on biological effects of simulated acid rain. Sci. Technol. Inf. 2008, 255, 255, 313. [Google Scholar]
- Sun, S.J.; Wen, Q.; Xie, P. Research advance on effects of acid rain on soil quality. J. Anhui Agri. Sci 2012, 40, 2774–2777. [Google Scholar]
- Fang, K.; Chen, X.M.; Du, Z.J.; Zhang, J.B.; Deng, J.Q.; Sun, J.H.; Zhou, C.M. SO42- and NO3- break through situations in representative red soils and prediction by Hydrus-1D model. J. Agro-Environ. Sci. 2009, 28, 107–111. [Google Scholar]
- Yang, X.; Xiang, C.G.; Liu, Z.X. Effect of heavy metal pollution on soil animals. Chin. Agric. Sci. Bull. 2008, 24, 454–457. [Google Scholar]
- Reinecke, A.J.; Reinecke, S.A.; Maboeta, M.S. Cocoon production and viability as endpoints in toxicity testing of heavy metals with three earthworm species. Pedobiologia 2001, 45, 61–68. [Google Scholar] [CrossRef]
- Salminen, J.; Haimi, J. The asexual enchytraeid worm Cognettia sphagnetorum (Oligochaeta) has increased Cu resistance in polluted soil. Environ. Pollut. 2001, 113, 221–224. [Google Scholar] [CrossRef]
- Guo, Y.C.; Wang, Z.Z.; Zhang, Y.M.; Lai, Q.; Yan, H.M.; Xia, W.S. Studies on toxicity and toxicology of heavy metals to earthworms in polluted soils. Chin. J. Appl. Environ. Biol 1996, 2, 132–140. [Google Scholar]
- Wang, Z.Z.; Zhang, Y.M.; Hu, J.L.; Zheng, Y.Y.; Hu, Z.Y.; Guo, Y.C.; Lai, Q.; Yan, H.M.; Deng, J.F. Effect of heavy metals in soil on earthworms (Opisthopora). Acya Sci. Circumstantiae 1994, 14, 236–247. [Google Scholar]
- Li, Z.X.; Huang, Y.X. Effect of suspension pH on structure and function of single living crocodilian erythrocyte. Chin. Sci. Bull. 2011, 2, 191–194. [Google Scholar]
- Shan, S.D.; Yu, J.Y.; Yu, W. Acid rain and soil ecosystem. Eco-Agric. Res. 2000, 8, 20–23. [Google Scholar]
- Chen, Q.H.; Sun, M.; Kuang, Q.; Shi, D.L.; Liu, H.; Hu, L.H.; Zhang, W.N.; Xia, D.; Zhu, Q.X. Influence of Cultivation Condition on Sporulation of Bacillus coagulans. Biotechnology 2009, 19, 77–81. [Google Scholar]
- Wang, J.L.; Zhou, X.P.; Sun, W. Effects of simulated acid rain on the release of aluminum ion in the soil. Anhui Agri. Sci. Bull. 2016, 22, 28–29, 120. [Google Scholar]
- Wang, C.Y. Effect of temperature and pH value on enzyme activity. Technol. Mark. 2016, 23, 232. [Google Scholar]
- Cheng, J.M.; Pan, G.X.; Cang, L.; Yang, J.J.; Wang, J.H. Effect of simulation acid rain on plant and pH of paddy soils in Taihu area. J. Nanjing Agric. Univ. 2000, 23, 116–118. [Google Scholar]
- Cen, H.X.; Wang, S.G.; Qiu, R.L.; Ma, L.F. Effect of simulating acid rain on cation release of some soils. Environ. Pollut. Control. 2001, 23, 13–15, 26. [Google Scholar]
- Li, Z.L.; Ding, H.T.; Yang, Y.Y.; Chen, X.J.; Zhao, Y.H.; Zhou, Q.F. Activities of cytochrome P450 enzyme CYP102A16 from Bacillus sp. C3. J. Zhejiang Univ. (Agric. Life Sci.) 2012, 38, 662–668. [Google Scholar]
- Gu, C.H.; Shan, Z.X. Simulation on releasing characteristics of Cd, Zn and Cu in soil polluted by acid rain. Min. Saf. Environ. Prot. 2010, 37, 6, 13–15, 19. [Google Scholar]













| pH | Accumulative Mortality Rate (%) |
|---|---|
| 2.0 | 100 |
| 2.5 | 40 |
| 3.0 | 0 |
| 3.5 | 0 |
| 4.0 | 0 |
| 4.5 | 0 |
| 5.0 | 0 |
| 5.5 | 0 |
| 7.0 | 0 |
| pH | Accumulative Mortality Rate (%) |
|---|---|
| 2.0 | 100 |
| 2.5 | 20 |
| 3.0 | 0 |
| 3.5 | 0 |
| 4.0 | 0 |
| 4.5 | 0 |
| 5.0 | 0 |
| 5.5 | 0 |
| 7.0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, J.; Wei, H. Physiological Responses of Earthworm Under Acid Rain Stress. Int. J. Environ. Res. Public Health 2020, 17, 7246. https://doi.org/10.3390/ijerph17197246
Chen X, Zhang J, Wei H. Physiological Responses of Earthworm Under Acid Rain Stress. International Journal of Environmental Research and Public Health. 2020; 17(19):7246. https://doi.org/10.3390/ijerph17197246
Chicago/Turabian StyleChen, Xuan, Jiaen Zhang, and Hui Wei. 2020. "Physiological Responses of Earthworm Under Acid Rain Stress" International Journal of Environmental Research and Public Health 17, no. 19: 7246. https://doi.org/10.3390/ijerph17197246