Removal of 1,4-Naphthoquinone by Birnessite-Catalyzed Oxidation: Effect of Phenolic Mediators and the Reaction Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. 1,4-NPQ and Birnessite
2.2. 1,4-NPQ Removal Experiments
2.3. Analytical Measurements
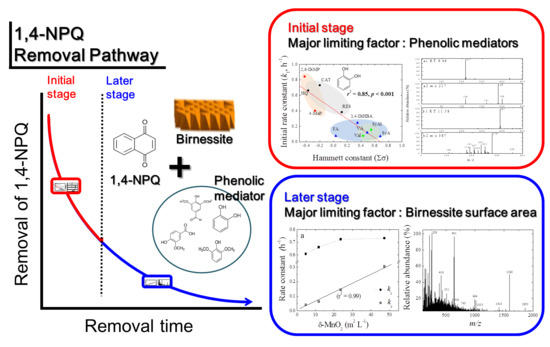
3. Results and Discussion
3.1. Effect of Phenolic Mediators on the Efficiency of 1,4-NPQ Removal by Birnessite
3.2. Cross-Coupling Reaction of 1,4-NPQ by Birnessite in the Presence of CAT
3.3. 1,4-NPQ Removal by Birnessite-Catalyzed Cross-Coupling with Distinct Reaction Stages
3.3.1. Effect of Birnessite Loading
3.3.2. Effect of CAT Loading and Reaction Sequence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.; Dong, D.; Meng, X.; Su, X.; Zheng, X.; Li, Y. Photolysis of polycyclic aromatic hydrocarbons on soil surfaces under UV irradiation. J. Environ. Sci. 2013, 25, 569–575. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Bigalke, M.; Kobza, J.; Wilcke, W. Sources and fate of polycyclic aromatic compounds (PAHs, oxygenated PAHs and azaarenes) in forest soil profiles opposite of an aluminium plant. Sci. Total Environ. 2018, 630, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Fu, L.; Cao, W.; Bai, Y.; Huang, Q.; Zhan, X. Occurrence and removal of polycyclic aromatic hydrocarbons and their derivatives in an ecological wastewater treatment plant in South China and effluent impact to the receiving river. Environ. Sci. Pollut. Res. 2019, 26, 5638–5644. [Google Scholar] [CrossRef]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, I.B.; Oberg, L.; Haglund, P.; Tyskind, M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. J. Hum. Environ. 2007, 36, 475–486. [Google Scholar] [CrossRef]
- Obrist, D.; Zielinska, B.; Perlinger, J.A. Accumulation of polycyclic aromatic hydrocarbons (PAHs) and oxygenated PAHs (OPAHs) in organic and mineral soil horizons from four U.S. remote forests. Chemosphere 2015, 134, 98–105. [Google Scholar] [CrossRef]
- Qiao, M.; Qi, W.; Liu, H.; Bai, Y.; Qu, J. Formation of oxygenated polycyclic aromatic hydrocarbons from polycyclic aromatic hydrocarbons during aerobic activated sludge treatment and their removal process. Chem. Eng. J. 2016, 302, 50–57. [Google Scholar] [CrossRef]
- Tidwell, L.G.; Blair Paulik, L.; Anderson, K.A. Air-water exchange of PAHs and OPAHs at a superfund mega-site. Sci. Total Environ. 2017, 603–604, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Sehlin, E. A study of the availability of PAHs and oxygenated PAHs in a contaminated soil. Ph.D. Thesis, Degree Project in Chemistry, Umea University, Umea, Sweden, 2004. [Google Scholar]
- Ferrarese, E.; Andreottola, G.; Oprea, I.A. Remediation of PAH-contaminated sediments by chemical oxidation. J. Hazard. Mater. 2008, 152, 128–139. [Google Scholar] [CrossRef]
- Woo, O.T.; Chung, W.K.; Wong, K.H.; Chow, A.T.; Wong, P.K. Photocatalytic oxidation of polycyclic aromatic hydrocarbons: Intermediates identification and toxicity testing. J. Hazard. Mater. 2009, 168, 1192–1199. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Knecht, A.L.; Goodale, B.C.; Truong, L.; Simonich, M.T.; Swanson, A.J.; Matzke, M.M.; Anderson, K.A.; Waters, K.M.; Tanguay, R.L. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 2013, 271, 266–275. [Google Scholar]
- Troester, M.A.; Lindstrom, A.B.; Waidyanatha, S.; Kupper, L.L.; Rappaport, S.M. Stability of hemoglobin and albumin adducts of naphthalene oxide, 1,2-Naphthoquinone, and 1,4-Naphthoquinone. Toxicol. Sci. 2002, 68, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Gurbani, D.; Bharti, S.K.; Kumar, A.; Pandey, A.K.; Ana, G.R.E.E.; Verma, A.; Khan, A.H.; Patel, D.K.; Mudiam, M.K.R.; Jain, S.K.; et al. Polycyclic aromatic hydrocarbons and their quinones modulate the metabolic profile and induce DNA damage in human alveolar and bronchiolar cells. Int. J. Hyg. Environ. Health. 2013, 216, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lim, D.M.; Lee, D.H.; Kang, K.H. Reaction kinetics and transformation products of 1-naphthol by Mn oxide-mediated oxidative-coupling reaction. J. Hazard. Mater. 2009, 165, 540–547. [Google Scholar] [CrossRef]
- Huguet, M.; Simon, V.; Gallard, H. Transformation of paracetamol into 1,4-benzoquinone by a manganese oxide bed filter. J. Hazard. Mater. 2014, 271, 245–251. [Google Scholar] [CrossRef]
- Shindo, H.; Huang, P.M. Role of Mn(IV) oxide in abiotic formation of humic substances in the environment. Nature 1982, 298, 363–365. [Google Scholar] [CrossRef]
- Chang, R.R.; Wang, S.L.; Liu, Y.T.; Chan, Y.T.; Hung, J.T.; Tzou, Y.M.; Tseng, K.J. Interactions of the products of oxidative polymerization of hydroquinone as catalyzed by birnessite with Fe (hydr)oxides – An implication of the reactive pathway for humic substance formation. RSC Adv. 2016, 6, 20750–20760. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Zhang, X.; Wang, Y.; Fan, J.Y.; Liu, Y.; Wang, N.; Chen, D.Y. Structural characteristics of humic-like acid from microbial utilization of lignin involving different mineral types. Environ. Sci. Pollut. Res. 2019, 26, 23923–23936. [Google Scholar]
- Song, Y.; Jiang, J.; Ma, J.; Zhou, Y.; von Gunten, U. Enhanced transformation of sulfonamide antibiotics by manganese(IV) oxide in the presence of model humic constituents. Water Res. 2019, 153, 200–207. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, W.; Wang, S.; Ge, J.; Qu, R.; Wang, Z. Oxidative oligomerization of phenolic endocrine disrupting chemicals mediated by Mn(III)-L complexes and the role of phenoxyl radicals in the enhanced removal: Experimental and theoretical studies. Environ. Sci. Technol. 2020, 54, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Dec, J.; Park, H.; Bollag, J.M. Effect of phenolic mediators and humic acid on cyprodinil transformation in presence of birnessite. Water Res. 2004, 38, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Bialk, H.M.; Simpson, A.J.; Pedersen, J.A. Cross-coupling of sulfonamide antimicrobial agents with model humic constituents. Environ. Sci. Technol. 2005, 39, 4463–4473. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.M. The synthesis of birnessite, cryptomelane, and some other oxides and hydroxides of manganese. Mineral. Mag. 1971, 38, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Chang Chien, S.W.; Chen, H.L.; Wang, M.C.; Seshaiah, K. Oxidative degradation and associated mineralization of catechol, hydroquinone and resorcinol catalyzed by birnessite. Chemosphere 2009, 74, 1125–1133. [Google Scholar] [CrossRef]
- Liu, L.; Tian, H.; He, J.; Wang, D.; Yang, Q. Preparation of birnessite-supported Pt nanoparticles and their application in catalytic oxidation of formaldehyde. J. Environ. Sci. 2012, 24, 1117–1124. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochim Acta—Part A. Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Takahata, Y.; Chong, D.P. Estimation of Hammett sigma constants of substituted benzenes through accurate density-functional calculation of core-electron binding energy shifts. Int. J. Quantum Chem. 2005, 103, 509–515. [Google Scholar] [CrossRef]
- Gan, W.; Ge, Y.; Zhu, H.; Huang, H.; Yang, X. ClO2 pre-oxidation changes the yields and formation pathways of chloroform and chloral hydrate from phenolic precursors during chlorination. Water Res. 2019, 148, 250–260. [Google Scholar] [CrossRef]
- Balgooyen, S.; Alaimo, P.J.; Remucal, C.K.; Ginder-Vogel, M. Structural transformation of MnO2 during the oxidation of bisphenol A. Environ. Sci. Technol. 2017, 51, 6053–6062. [Google Scholar] [CrossRef]
- Shindo, H. Catalytic synthesis of humic acids from phenolic compounds by Mn(IV) oxide (birnessite). Soil Sci. Plant Nutr. 1990, 36, 679–682. [Google Scholar] [CrossRef]
- Kennedy, B.; Glidle, A.; Cunnane, V.J. A study of the oxidation and polymerisation of meta substituted phenol and aniline derivatives. J. Electroanal. Chem. 2007, 608, 22–30. [Google Scholar] [CrossRef]
- García Einschlag, F.S.; Carlos, L.; Capparelli, A.L. Competition kinetics using the UV/H2O2 process: A structure reactivity correlation for the rate constants of hydroxyl radicals toward nitroaromatic compounds. Chemosphere 2003, 53, 1–7. [Google Scholar] [CrossRef]
- Choi, C.K.; Eom, W.S.; Shin, H.S. Effect of phenolic mediators and humic acid on the removal of 1-indanone using manganese oxide. J. Korean Soc. Environ. Eng. 2012, 34, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Li, S.; Waigi, M.G.; Huang, Q. Nano-MnO2-mediated transformation of triclosan with humic molecules present: Kinetics, products, and pathways. Environ. Sci. Pollut. Res. 2018, 25, 14416–14425. [Google Scholar] [CrossRef]
- Pillar, E.A.; Zhou, R.; Guzman, M.I. Heterogeneous oxidation of catechol. J. Phys. Chem. A 2015, 119, 10349–10359. [Google Scholar] [CrossRef]
- Lin, K.; Liu, W.; Gan, J. Oxidative removal of bisphenol A by manganese dioxide: Efficacy, products, and pathways. Environ. Sci. Technol. 2009, 43, 3860–3864. [Google Scholar] [CrossRef]
- Majcher, E.H.; Chorover, J.; Bollag, J.M.; Huang, P.M. Evolution of CO2 during birnessite-induced oxidation of 14C-labeled catechol. Soil Sci. Soc. Am. J. 2000, 64, 157–163. [Google Scholar] [CrossRef]
- Park, J.W.; Dec, J.; Kim, J.E.; Bollag, J.M. Effect of humic constituents on the transformation of chlorinated phenols and anilines in the presence of oxidoreductive enzymes or birnessite. Environ. Sci. Technol. 1999, 33, 2028–2034. [Google Scholar] [CrossRef]
- Remucal, C.K.; Ginder-Vogel, M. A critical review of the reactivity of manganese oxides with organic contaminants. Environ. Sci. Process. Impacts 2014, 16, 1247–1266. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Huang, Q.; Mao, L. Removal of acetaminophen using enzyme-mediated oxidative coupling processes: I. Reaction rates and pathways. Environ. Sci. Technol. 2009, 43, 7062–7067. [Google Scholar] [CrossRef]
- Ulrich, H.J.; Stone, A.T. Oxidation of chlorophenols adsorbed to manganese oxide surfaces. Environ. Sci. Technol. 1989, 23, 421–428. [Google Scholar] [CrossRef]
- Zhang, H. Metal oxide-facilitated oxidation of antibacterial agent. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2004. [Google Scholar]
- Pal, S.; Bollag, J.M.; Huang, P.M. Role of abiotic and biotic catalysts in the transformation of phenolic compounds through oxidative coupling reactions. Soil Biol. Biochem. 1994, 26, 813–820. [Google Scholar] [CrossRef]





| Phenolic Mediator | RT (min) a | Efficiency (%) b | Kinetic Parameters | ||
|---|---|---|---|---|---|
| k1 c (× 10−1, h−1) | k2 d (× 10−1, h−1) | ||||
| None (control) e | - | 1.7 ± 0.1 | <0.001 | <0.001 | |
| δ-MnO2 only f | - | 9.8 ± 0.8 (3.0) | 0.28 (0.81) | 0.03 (0.89) | |
| Diphenol | Catechol (CAT) | 1.83 | 100 ± 0.3 (82.3) | 7.33 (0.98) | 4.83 (0.98) |
| Hydroquinone (HQ) | 2.18 | 100 ± 0.4 (65.0) | 6.11 (0.98) | 4.08 (0.98) | |
| Resorcinol (RES) | 1.75 | 100 ± 0.8 (46.7) | 3.80 (0.98) | 3.66 (0.98) | |
| Methoxy phenol | 4-Methoxyphenol (4-MeP) | 2.25 | 99.8 ± 0.4 (47.6) | 4.00 (0.98) | 4.03 (0.99) |
| 2,6-Dimethoxyphenol (2,6-DiMeP) | 2.36 | 99.9 ± 0.5 (71.6) | 6.57 (0.98) | 6.16 (0.98) | |
| Acidic phenol | Syringic acid (SyA) | 2.61 | 77.6 ± 1.2 (15.9) | 0.71 (0.96) | 0.64 (1.00) |
| Vanillic acid (VA) | 2.38 | 79.5 ± 0.5 (22.6) | 1.18 (0.97) | 1.11 (1.00) | |
| 3,4-Dihydroxybenzoic acid (3,4-DiHBA) | 2.33 | 94.9 ± 0.7 (38.7) | 2.44 (0.97) | 1.51 (1.00) | |
| Ferulic acid (FA) | 2.46 | 93.6 ± 0.8 (15.5) | 0.75 (0.98) | 0.59 (1.00) | |
| Aldehyde phenol | Syringic aldehyde (SyAl) | 2.61 | 98.1 ± 1.2 (27.3) | 1.56 (0.98) | 1.60 (1.00) |
| Vanillin (Val) | 2.25 | 87.8 ± 1.1 (16.4) | 0.79 (0.96) | 0.80 (1.00) | |
| Birnessite loading (g L−1) a | ||||
| 0.1 | 0.25 | 0.5 | 1.0 | |
| k1 (h−1) b | 00.608 | 00.651 | 0.723 | 0.731 |
| r2 | 00.990 | 00.990 | 0.990 | 0.990 |
| t1/2 (h) | 01.140 | 01.060 | 0.960 | 0.950 |
| k2 (h−1) c | 00.041 | 00.063 | 0.143 | 0.304 |
| r2 | 00.990 | 00.990 | 0.990 | 0.990 |
| t1/2 (h) | 16.900 | 11.000 | 4.850 | 2.280 |
| Ksurf (L m−2 h−1) d | 6.36 × 10−4 | |||
| CAT loading (mM) e | ||||
| 0.1 | 0.3 | 0.5 | 1.0 | |
| k1 (h−1) b | 00.631 | 00.711 | 0.847 | 0.997 |
| r2 | 00.990 | 00.980 | 0.990 | 0.980 |
| t1/2 (h) | 01.100 | 00.990 | 0.820 | 0.700 |
| Kcat (L mM−1 h−1) f | 0.383 | |||
| k2 (h−1) c | 00.154 | 00.144 | 0.156 | 0.133 |
| r2 | 00.960 | 00.960 | 0.980 | 0.980 |
| t1/2 (h) | 04.500 | 04.810 | 4.440 | 5.210 |
| Birnessite Loading (g L−1) a | Reaction Sequence | k1 (h−1) b | r2 | t1/2 (h) | k2 (h−1) c | r2 | t1/2 (h) |
|---|---|---|---|---|---|---|---|
| 0.5 | Batch 1 d | 0.847 | 0.97 | 0.82 | 0.156 | 0.94 | 4.44 |
| Batch 2 e | 0.389 | 0.96 | 1.78 | 0.159 | 0.95 | 4.35 | |
| 1.0 | Batch 1 d | 0.935 | 0.96 | 0.74 | 0.298 | 0.94 | 2.33 |
| Batch 2 e | 0.555 | 0.97 | 1.25 | 0.274 | 0.95 | 2.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Hur, J.; Lee, D.-H.; Schlautman, M.A.; Shin, H.-S. Removal of 1,4-Naphthoquinone by Birnessite-Catalyzed Oxidation: Effect of Phenolic Mediators and the Reaction Pathway. Int. J. Environ. Res. Public Health 2020, 17, 4853. https://doi.org/10.3390/ijerph17134853
Lee H-S, Hur J, Lee D-H, Schlautman MA, Shin H-S. Removal of 1,4-Naphthoquinone by Birnessite-Catalyzed Oxidation: Effect of Phenolic Mediators and the Reaction Pathway. International Journal of Environmental Research and Public Health. 2020; 17(13):4853. https://doi.org/10.3390/ijerph17134853
Chicago/Turabian StyleLee, Han-Saem, Jin Hur, Doo-Hee Lee, Mark A. Schlautman, and Hyun-Sang Shin. 2020. "Removal of 1,4-Naphthoquinone by Birnessite-Catalyzed Oxidation: Effect of Phenolic Mediators and the Reaction Pathway" International Journal of Environmental Research and Public Health 17, no. 13: 4853. https://doi.org/10.3390/ijerph17134853