Investigation of the Pigments and Glassy Matrix of Painted Enamelled Qing Dynasty Chinese Porcelains by Noninvasive On-Site Raman Microspectrometry
Abstract
:1. Introduction
2. Methods and Artifacts
2.1. Artifacts
2.2. Mobile Raman Microspectrometry
2.3. Mobile X-ray Fluorescence Microspectrometry
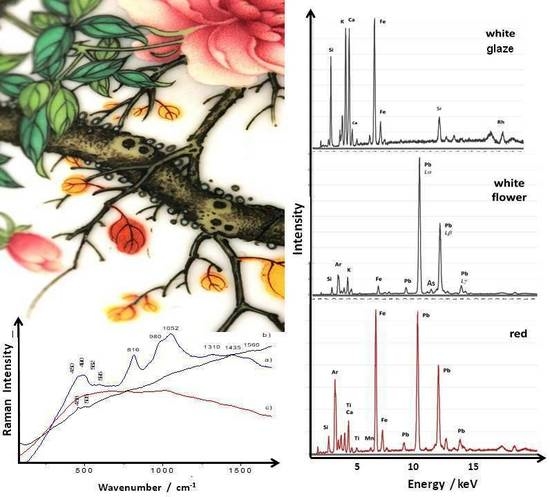
3. Results and Discussion
3.1. Body Phase Identification
3.2. Phase Identification in Colored Glaze/Enamels
3.2.1. Arsenic-Based Phases
3.2.2. Lead Pyrochlore (Naples Yellow)
3.2.3. Red to Violet Colors
3.3. Glassy Matrix
3.4. XRF Analysis
4. Comparison with Painted Enameled Metalware
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montanari, R.; Alberghina, M.F.; Casanova Municchia, A.; Massa, E.; Pelagotti, A.; Pelosi, C.; Schiavone, S.; Sodo, A. A polychrome Mukozuke (1624–1644) porcelain offers a new hypothesis on the introduction of European enameling technology in Japan. J. Cult. Herit. 2018, 32, 232–237. [Google Scholar] [CrossRef]
- Montanari, R.; Murakami, N.; Alberghina, M.F.; Pelosi, C.; Schiavone, S. The Origin of overglaze-blue enameling in Japan: New discoveries and a reassessment. J. Cult. Herit. 2019, 37, 94–102. [Google Scholar] [CrossRef]
- Montanari, R.; Murakami, N.; Colomban, P.; Alberghina, M.F.; Pelosi, C.; Schiavone, S. European Ceramic technology in the Far East: Enamels and pigments in Japanese art from the 16th to the 20th century and their reverse influence on China. Herit. Sci. 2020, 8, 48. [Google Scholar] [CrossRef]
- Shih, C.F. Evidence of East-West exchange in the eighteenth century: The establishment of painted enamel art at the Qing Court in the reign of Emperor Kangxi. Natl. Palace Mus. Res. Quaterly 2007, 24, 45–94. [Google Scholar]
- Zhou, S.Z. Research on Painted Enamels Porcelain Ware from the Qing Court; Wenwu Chubanshe: Beijing, China, 2008. [Google Scholar]
- Lili, F. La Céramique Chinoise; China Intercontinental Press: Beijing, China, 2011. [Google Scholar]
- Xu, X.D. Europe-China-Europe: The Transmission of the Craft of Painted Enamel in the Seventeenth and Eighteenth Centuries. In Goods from the East, 1600–1800 Trading Eurasia; Berg, M., Ed.; Houndmills, Basingstoke, Palgrave Macmillan: Hampshire, UK, 2015; pp. 92–106. [Google Scholar]
- Zhao, B.; Wang, G.; Biron, I.; Colomban, P.; Hilaire-Pérez, L. La circulation des techniques de l’émail entre la France et la Chine du XVIIème au XIXème siècle. Le CNRS en Chine Bulletin 2016, 21, 21–25. Available online: http://www.cnrs.fr/derci/IMG/pdf/cnrsenchine_21_fr_final_pour_le_site_cnrs.pdf (accessed on 23 July 2020).
- Zhou, L.L. Discussion on Falangcai Enamels-and the Difference between Falangcai and Yangcai. Shangai Bowuguan Jikan 2000, 8, 210–226. [Google Scholar]
- Kırmızı, B.; Colomban, P.; Quette, B. On-site analysis of Chinese Cloisonné enamels from fifteenth to nineteenth centuries. J. Raman Spectrosc. 2010, 41, 780–790. [Google Scholar]
- Colomban, P.; Arberet, L.; Kırmızı, B. On-Site Raman Analysis of 17th and 18th Century Limoges Enamels: Implications on the European Cobalt Sources and the Technological Relationship between Limoges and Chinese Enamels. Ceram. Int. 2017, 43, 10158–10165. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Zhang, Y.; Zhao, B. Non-invasive Raman analyses of huafalang and related porcelain wares. Searching for evidence for innovative pigment technologies. Ceram. Int. 2017, 43, 12079–12088. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Ambrosi, F.; Ngo, A.-T.; Lu, T.-A.; Feng, X.-L.; Chen, S.; Choi, C.-L. Comparative analysis of wucai Chinese porcelains using mobile and fixed Raman microspectrometers. Ceram. Int. 2017, 43, 14244–14256. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B.; Yang, Y. Non-invasive on-site Raman study of pigments and glassy matrix of the 17th–18th century painted enamelled Chinese metal wares: Comparison with French enamelling technology. Coatings 2020, 10, 471. [Google Scholar] [CrossRef]
- Giannini, R.; Freestone, I.C.; Shortland, A.J. European cobalt sources identified in the production of Chinese famille rose porcelain. J. Archaeol. Sci. 2017, 80, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Kırmızı, B. Non-invasive on-site Raman study of polychrome and white enamelled glass artefacts in imitation of porcelain assigned to Bernard Perrot and his followers. J. Raman Spectrosc. 2020, 51, 133–146. [Google Scholar] [CrossRef]
- Colomban, P.; Lu, T.-A.; Milande, V. Non-invasive on-site Raman study of blue-decorated early soft-paste porcelain: The use of arsenic-rich (European) cobalt ores—Comparison with huafalang Chinese porcelains. Ceram. Int. 2018, 44, 9018–9026. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th-18th century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cult. Herit. 2020. [Google Scholar] [CrossRef]
- Kingery, W.D.; Vandiver, P.B. The Eighteenth-Century Change in Technology and Style from the Famille-Verte Palette to the Famille-Rose Palette. In Technology and Style; Kingery, W.D., Ed.; Ceramics and Civilization Serie; The American Ceramic Society: Colombus, OH, USA, 1986; Volume 2, pp. 363–381. [Google Scholar]
- Colomban, P. On-site Raman study of artwork: Procedure and illustrative examples. J. Raman Spectrosc. 2018, 49, 921–934. [Google Scholar] [CrossRef]
- Colomban, P. The on-site/remote Raman analysis with mobile instruments: A review of drawbacks and success in cultural heritage studies and other associated fields. J. Raman Spectrosc. 2012, 43, 1529–1535. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Edwards, H.G.M.; Jehlička, J. The role of mobile instrumentation in novel applications of Raman spectroscopy: Archaeometry, geosciences, and forensics. Chem. Soc. Rev. 2014, 43, 2628–2649. [Google Scholar] [CrossRef]
- Madariaga, J.M. Analytical chemistry in the field of cultural heritage. Anal. Methods 2015, 7, 4848–4876. [Google Scholar] [CrossRef]
- Simsek, G.; Unsalan, O.; Bayraktar, K.; Colomban, P. On-site pXRF analysis of glaze composition and colouring agents of “Iznik” tiles at Edirne mosques (15th and 16th-centuries). Ceram. Int. 2019, 45, 595–605. [Google Scholar] [CrossRef]
- Defretin, J. La Datation de la Porcelaine “Coquille D’euf” D’exportation du Musée du Louvre, Mémoire, 2nd Cycle—Histoire de L’art Appliquée Aux Collections; Ecole du Louvre: Paris, France, 2020. [Google Scholar]
- Howard, D.S. Chinese Armorial Porcelain; Heirlomm & Howard Ltd.: London, UK, 2003; Volume 2, p. 155. [Google Scholar]
- Tang, H. ‘The Colours of Each Piece’: Production and consumption of Chinese Enamelled Porcelain, c.1728-c.1780. Ph.D. Thesis, Warwick University, Warwick, UK, March 2017. Available online: http://wrap.warwick.ac.uk/91791 (accessed on 26 June 2020).
- Colomban, P.; Treppoz, F. Identification and differentiation of ancient and modern European porcelains by Raman macro and micro-spectroscopy. J. Raman Spectrosc. 2001, 32, 93–102. [Google Scholar] [CrossRef]
- Colomban, P.; Sagon, G.; Faurel, X. Differentiation of antique ceramics from the Raman spectra of their coloured glazes and paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Carty, W.M.; Senapati, U. Porcelain-raw materials, processing, phase evolution, and mechanical behavior. J. Am. Ceram. Soc. 1998, 81, 3–20. [Google Scholar] [CrossRef]
- Sciau, P.; Noé, L.; Colomban, P. Metal nanoparticles in contemporary potters’ masterpieces: Lustre and red “pigeon blood” potteries as models to understand the ancient pottery. Ceram. Int. 2016, 42, 15349–15357. [Google Scholar] [CrossRef]
- Manoun, B.; Azdouz, M.; Azrour, M.; Essehli, R.; Benmokhtar, S.; El Ammari, L.; Ezzahi, A.; Ider, A.; Lazor, P. Synthesis, Rietveld refinements and Raman spectroscopic studies of tricationic lacunar apatites Na1−xKxPb4(AsO4)3 (0 < x < 1). J. Mol. Struct. 2011, 986, 1–9. [Google Scholar]
- Van Pevenage, J.; Lauwers, D.; Herremans, D.; Verhaeven, E.; Vekemans, B.; De Clercq, W.; Vincze, L.; Moens, L.; Vandenabeele, P. A combined spectroscopic study on Chinese porcelain containing ruan-cai colours. Anal. Methods 2014, 6, 387–394. [Google Scholar] [CrossRef]
- Colomban, P.; Maggetti, M.; d’Albis, A. Non-invasive Raman identification of crystalline and glassy phases in a 1781 Sèvres Royal Factory soft paste porcelain plate. J. Eur. Ceram. Soc. 2018, 38, 5228–5233. [Google Scholar] [CrossRef]
- Kissin, S.A. Five element (Ni-Co-As-Ag-Bi) veins. Geosci. Can. 1992, 19, 113–124. [Google Scholar]
- Colomban, P. Rocks as blue, green and black pigments/dyes of glazed pottery and enamelled glass artefacts—A review. Eur. J. Mineral. 2014, 25, 863–879. [Google Scholar] [CrossRef]
- Berrie, B.H. Mining for Color: New Blues, Yellows, and Translucent Paint. Early Sci. Med. 2015, 20, 308–334. [Google Scholar] [CrossRef] [Green Version]
- Pradell, T.; Molina, G.; Molera, J.; Pla, J.; Labrador, A. The use of micro-XRD for the study of glaze color decorations. Appl. Phys. A 2013, 111, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Viti, C.; Borgia, I.; Brunetti, B.; Sgamellotti, A.; Mellini, M. Microtexture and microchernistry of glaze and pigments in Italian Renaissance pottery from Gubbio and Deruta. J. Cult. Herit. 2003, 4, 199–210. [Google Scholar] [CrossRef]
- Pérez-Arantegui, J.; Resano, M.; Garcia-Ruiz, E.; Vanhaecke, F.; Roldan, C.; Ferrero, J.; Coll, J. Characterization of cobalt pigments found in traditional Valencian ceramics by means of laser ablation-inductively coupled plasma mass spectrometry and portable X-ray fluorescence spectrometry. Talanta 2008, 74, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arantegui, J.; Montull, B.; Resano, M.; Ortega, J.M. Materials and technological evolution of ancient cobalt-blue-decorated ceramics: Pigments and work patterns in tin-glazed objects from Aragon (Spain) from the 15th to the 18th century AD. J. Eur. Ceram. Soc. 2009, 29, 2499–2509. [Google Scholar] [CrossRef]
- Colomban, P.; Sagon, G.; Huy, L.Q.; Liem, N.Q.; Mazerolles, L. Vietnamese (15th century) blue-and-white, tam thai and “luster” porcelains/stoneware: Glaze composition and decoration techniques. Archaeometry 2004, 46, 125–136. [Google Scholar] [CrossRef]
- Figueiredo, M.O.; Silva, T.P.; Veiga, J.P. A XANES study of cobalt speciation state in blue-and-white glazes from 16th to 17th century Chinese porcelains. J. Electr. Spectrosc. Relat. Phenom. 2012, 185, 97–102. [Google Scholar] [CrossRef]
- Dias, M.I.; Prudêncio, M.I.; de Matos, M.A.P.; Rodrigues, A.L. Tracing the origin of blue and white Chinese Porcelain ordered for the Portugese market during the Ming dynasty using INAA. J. Archaeol. Sci. 2013, 40, 3046–3057. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P.; Wong, S.; Zhao, B.; Rougeulle, A.; Liem, N.Q. Toward a fast non-destructive identification of pottery: The sourcing of 14th-16th century Vietnamese and Chinese ceramic shards. J. Cult. Herit. 2015, 16, 159–172. [Google Scholar] [CrossRef]
- Fischer, C.; Hsieh, E. Export Chinese Blue-and-white porcelain: Compositional analysis and sourcing using non-invasive portable XRF and reflectance spectroscopy. J. Archaeol. Sci. 2016, 80, 14–26. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S. Lead-tin-antimony yellow-Historical manufacture, molecular characterization and identification in seventeenth-century Italian paintings. Stud. Conserv. 2004, 49, 41–52. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S.; Lopez-Gil, A.; Miralles, J. Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery. J. Raman Spectrosc. 2006, 37, 1146–1153. [Google Scholar] [CrossRef]
- Pereira, M.; de Lacerda-Aroso, T.; Gomes, M.J.M.; Mata, A.; Alves, L.C.; Colomban, P. Ancient Portuguese ceramic wall tiles (Azulejos): Characterization of the glaze and ceramic pigments. J. Nano Res. 2009, 8, 79–88. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Pelosi, C.; Agresti, G.; Santamaria, U.; Mattei, E. Artificial yellow pigments: Production and characterization through spectroscopic methods of analysis. E-Preserv. Sci. 2010, 7, 108–115. [Google Scholar]
- Kirmizi, B.; Colomban, P.; Blanc, M. On-site Analysis of Limoges enamels from 16th to 19th centuries. J. Raman Spectrosc. 2010, 41, 1240–1247. [Google Scholar] [CrossRef]
- Dik, J.E.; Hermens, R.; Peschar, H. Schenk, Early production recipes for lead antimonite yellow in Italian art. Archaeometry 2005, 47, 593–607. [Google Scholar] [CrossRef]
- Dik, J. Scientific analysis of historical paint and the implications for art history and art conservation. The case studies of naples yellow and discoloured smalt. Doctorate Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2003. Available online: https://dare.uva.nl/search?identifier=98d80b7d-a63e-450b-a984-611a3d0c0cfe (accessed on 14 August 2020).
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman spectroscopic library of natural an synthetic pigments (pre-~1850 AD). Spectrochim. Acta Part A 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Colomban, P. The destructive/non-destructive identification of enamelled pottery and glass artifacts and associated pigments—A brief overview. Arts 2013, 2, 77–110. [Google Scholar] [CrossRef] [Green Version]
- Neri, E.; Morvan, C.; Colomban, P.; Guerra, M.P. Prigent, Late Roman and Byzantine Mosaic opaque Glass-ceramics Tesserae (5th–9th century). Ceram. Int. 2016, 42, 18859–18869. [Google Scholar] [CrossRef] [Green Version]
- Rosi, F.; Manueli, V.; Grygar, T.; Bezdicka, P.; Brunetti, B.G.; Sgamelotti, A.; Burgio, L.; Seccaronif, C.; Miliani, C. Raman scattering features of lead pyroantimonate compounds: Implication for the non-invasive identification of yellow pigments on ancient ceramics. Part II. In situ characterisation of Renaissance plates by portable micro-Raman and XRF studies. J. Raman Spectrosc. 2011, 42, 407–414. [Google Scholar] [CrossRef]
- Cartechini, L.; Rosi, F.; Miliani, C.; D’Acapito, F.; Brunetti, B.G.; Sgamellotti, A. Modified Naples yellow in Renaissance majolica: Study of Pb-Sb-Zn and Pb-Sb-Fe ternary pyroantimonates by X-ray absorption spectroscopy. J. Anal. Atom. Spectrom. 2011, 26, 2500–2507. [Google Scholar] [CrossRef]
- Biron, I.; Chopinet, M.-H. Colouring, Decolouring and Opacifying Glass. In Modern Methods for Analysing. Archaeological and Historical Glass, 1st ed.; Janssens, K., Ed.; John Wiley & Sons Ltd.: London, UK, 2012; pp. 49–66. [Google Scholar]
- Verita, M.; Maggetti, M.; Sagui, L.; Santopadre, P. Colors of Roman Glass: An Investigation of the Yellow Sectilia in the Gorga Collection. J. Glass Stud. 2013, 55, 21–34. [Google Scholar]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Owens, F.J.; Orosz, J. Effect of nanosizing on lattice and magnon modes of hematite. Solid State Commun. 2006, 138, 95–98. [Google Scholar] [CrossRef]
- Hunt, L.B. The true story of Purple of Cassius. Gold Bull. 1976, 9, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P. The Use of Metal Nanoparticles to Produce Yellow, Red and Iridescent Colour, from Bronze Age to Present Times in Lustre Pottery and Glass: Solid State Chemistry, Spectroscopy and Nanostructure. J. Nano Res. 2009, 8, 109–132. [Google Scholar] [CrossRef] [Green Version]
- Geyssant, J. Secret du verre rouge transparent de Bernard Perrot et comparaison avec celui de Johann Kunckel. In Bernard Perrot (1640-1709): Secrets et Chefs-d’œuvre des Verreries Royales d’Orléans, Catalogue; Musée des Beaux-Arts d’Orléans, SOMOGY Editions d’Arts: Paris, France, 2010; pp. 51–54. [Google Scholar]
- Louis, C. Gold nanoparticles in the past: Before the Nanotechnology Era. In Gold Nanoparticles for Physics, Chemistry and Biology; Louis, C., Pluchery, O., Eds.; Imperial College Press: London, UK, 2012; pp. 1–27. [Google Scholar]
- Colomban, P. Polymerisation Degree and Raman Identification of Ancient Glasses used for Jewellery, Ceramics Enamels and Mosaics. J. Non-Cryst. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A.; Bellot-Gurlet, L. Raman Identification of glassy silicates used in ceramic, glass and jewellry: A tentative differentiation guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Paulsen, O. Non-destructive Raman Determination of the Structure and Composition of Glazes by Raman Spectroscopy. J. Am. Ceram. Soc. 2005, 88, 390–395. [Google Scholar] [CrossRef]
- Colomban, P. Non-Destructive Raman Analysis of Ancient Glasses and Glazes, in Modern Methods for Analysing Archaeological and Historical Glass, 1st ed.; Janssens, K., Ed.; John Wiley & Sons Ltd.: London, UK, 2012; pp. 275–300. [Google Scholar]
- Labet, V.; Colomban, P. Vibrational properties of silicates: A cluster model able to reproduce the effect of “SiO4” polymerization on Raman intensities. J. Non-Cryst. Solids 2013, 370, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Qu, L.; Duan, H.; Tarcea, N.; Shen, A.; Popp, J.; Hu, J. Elemental analysis-aided Raman spectroscopic studies on Chinese cloisonné wares and painted enamels from the Imperial palace. Spectrochim. Acta A-Mol. Biomol. Spectrosc. 2016, 153, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Tregear, M.; Wood, N. The technology of sixteenth- and seventeenth century Chinese Cloisonné enamels. Archaeometry 1989, 31, 133–146. [Google Scholar] [CrossRef]
- Shih, C.F. Radiant Luminance: The Painted Enamelware of the Qing Imperial Court; The National Palace Museum of Taipei: Taipei, Taiwan, 2012. [Google Scholar]
- Wood, N. Chinese Glazes: Their Origins, Chemistry and Recreation; A & C Black: London, UK, 1999. [Google Scholar]
- Shi, N.C. Analyse Scientifique des Couleurs Falang D’objets Conservés au Palais ou Retrouvés en Contexte Archéologique. In Proceedings of the French-Chinese Meeting on Enamelling Technology, Collège de France, Paris, France, 10 September 2019. [Google Scholar]
















| Artifacts | Inventory Number | Collection | Artifacts | Inventory Number | Size | Artifacts |
|---|---|---|---|---|---|---|
 | R1041 cup | Baronne Salomon de Rothschild bequest | Louvre Museum | 1722–1735 | H:3.9 D: 7.2 | Similar cups at the British Museum (FRANKS 703), at the Fitzwilliam Museum (OC.29-1936) and at the Art Gallery of Greater Victoria (1991.014.021) |
| R1045 cup | 1730–1745 | H:4 D:7 | Similar cup saucer at Rijksmuseum (AK-NM-13720-A) [25] | |||
| R1048 cup | 1740–1760 | H:4 D:7 | China, Guangzhou workshop? | |||
| R1135 cup | ca. 1750–1760 | H:3.7 D:6.6 | China, Guangzhou workshop? | |||
 | R1006 dish | 1740–1760? | D~10 | |||
 | R1177 saucer lid | D:11.5 | Series with the coat of arms of an English family, S. Jones de Stepney or J. Bromwell Jones or his son or brother [25,26]. | |||
 | SN284 milk pot | ca. 1735 | H:11 D:6.8 | |||
 | R1056 plate | 1722–1735 | D:19 | With 3 tigers as decor | ||
| R1175 (2) plate | 1735 | H:1.7 L:13.2 | With the coat of arms of an English family (see above) | |||
 | R1025 dish | 1740–1750 | H:3.2 D:23 | With CFB monogram | ||
 | TH487 dish | A. Thiers Coll. | 1730–1735 | With 2 roosters drawn | ||
 | TH457 bottle | A. Thiers Coll. | 1735–1796 | H:30.9 D:12 | ||
 | F1371C bottle | Napoléon Emperor Coll. | Fontaine bleau Castle (Musée Chinois) | 1730/35–1750/60 | H:19 D:30 | |
 | F1429C teapot | Qianlong period (1736–1795) | H:13 D:12 A:19 | Jingdezhen Imperial Factory of Porcelain | ||
 | F1341C bottle | End of Yongzheng period (~1730–1735) | H:30 D:15 |
| Color | Artifact | Identified Phase(s) | Characteristic Raman Peak (cm−1) | Remarks |
|---|---|---|---|---|
| White (colorless glaze) | R1006, R1041, R1056, R1175, TH457, TH487 | quartz | 455–460 | |
| glassy aluminosilicate | ~485 (br) | |||
| White (overglaze) | F1371C, R1048, R1056 | As apatite | ~813 (nr), ~770 (sh) | White type 1 |
| White-pink (overglaze) | TH457 | arsenate | 820 (br) | White type 2 |
| Blue (overglaze) | R1175 (s), R1135, F1371C, F1429C | As apatite | ~810 (nr), ~775 (sh) | Blue type 1 |
| TH487, R1045, R1048, R1041 | arsenate | ~815 (br) | Blue type 2 | |
| Blue (glaze) | R1006 | glassy silicate (+ quartz) | 460, 503 | Blue type 3 Smalt? |
| Yellow (overglaze) | R1175, TH457 | Sb-rich NY pyrochlore | ~122/130, ~335, 508 | |
| R1175, R1006, R1048, TH487, SN284, R1135 | Sn-rich NY pyrochlore | ~130–133, ~455 | ||
| TH487, R1056 | Sn-rich NY pyrochlore + arsenate | ~133, 327, 440, ~810–820 (broad) | ||
| F1429C, F1341C?, TH457 | Sb–Sn–(Zn, Fe?) NY pyrochlore + As apatite | 134, 330, 450, 480–490, 518, 810, 770 (sh) | ||
| Yellow-green (overglaze) | R1135 | Sn-rich NY pyrochlore + As-apatite | ~133, 330, 815, 770 | |
| Green (overglaze) | R1135 | Sn-rich NY pyrochlore | ~130–133, 330, 455 | |
| Blue-green (overglaze) | TH487 | Sn-rich NY pyrochlore + arsenate | ~133, 330, 440, ~810 | |
| Dark yellow (overglaze) | TH457 | Feldspar? + hematite | ~508 + 220, 292, ~1310 | |
| Red (overglaze) | TH457, R1175, R1048, R1135 | hematite | 220, 292, ~1310 | |
| SN284, R1175 (large), TH487 | Au° + arsenate | Fluorescence background peaking at ~500 + ~810 | ||
| Orange (overglaze) | TH457, SN284 | Au° | Fluorescence background peaking at ~600 | |
| R1175, R1041, TH487 | Au° + arsenate | Fluorescence background + ~820 | ||
| R1175 | hematite | 220, 290, ~1305 | ||
| Pink (rose) (overglaze) | SN284 | Arsenate + Au°? | ~810 + fluorescence background at ~500 | |
| R1041, F1429C | Au° | Fluorescence background at ~500 | ||
| Black (overglaze) | TH487 | Mn oxide | 470–575 | |
| R1175, R1048, TH487 | Mn spinel/oxide (+ carbon) | 502–605 (+1330–1570) | ||
| R1006 | Spinel (Mn?) (+ carbon) | 625 (+1330–1565) | ||
| Brown | F1429C | Mn oxide | 550, 580, 892 |
| Artifact | Si–O Stretching Components (cm−1) | Color | Glass Type | Observed in Cloisonné Metalware [14] | Observed in Painted Metalware [14] |
|---|---|---|---|---|---|
| F1371C | 1025, 1130 | White, blue | L-a IIa | Blue and white | Not observed except F1440C |
| R1048 | 980, 1040 975, 1040 1075 | White Blue Black, yellow, red | L-a IIa L-a IIa L-a III | ||
| R1056 | 980, 1035 | White | L-a IIa | ||
| TH457 | 985, 1130 985 1070 | White pink Yellow Dark yellow, red, blue | L-rich I L-rich I L-a III | Yellow and black Green-yellow | Yellow and turquoise yellow and green |
| R1175 | 985, 1040 975 985, 1040 905, 970, 1035, 1120 | Blue Yellow Black Green, orange, red | L-a IIa L-rich I L-a IIb L-a IIa | See above See above Green | Green |
| TH487 | 975, 1050 970, 1045 920, 1140 915, 971, 1037 | Blue Yellow Black Green, red | L-a IIa L-a IIa L-rich I L-a IIb | See above | |
| R1045 | 975, 1035 | Blue, white | L-a IIb | ||
| R1041 | 980, 1050 | Blue, pink | L-a IIa | ||
| R1006 | 1015 1020 | Yellow Black | L-rich I L-rich I | ||
| SN284 | 980, 1060 | Yellow, red, pink | L-a IIa | ||
| R1135 | 1020 | Green, blue, red | L-rich I | ||
| F1341C | 970, 1035 980, 1030 1040, 1090 | White, blue Green Red | L-a IIb L-a IIb L-a IIa | ||
| F1429C | 980, 1030, 1140 970, 1020 900, 975, 1010, 1130 900, 975, 1010, 1125 | White, blue Light pink Yellow Green | L-a IIa L-a IIa L-rich I L-a IIb |
| Glaze | Elements |
|---|---|
| White | Si, K, Ca, Fe–Sr |
| Overglaze enamel | |
| Blue | Si, Pb–K, Co, Fe, As–Ni, Mn |
| White | Si, Pb–K, Fe, Ca, As–Ni |
| Green | Si, Pb–Cu, Fe, K, Ca, Sn, Zn–Mn, Ni, (Co?) |
| Yellow | Si, Pb–K, Sb, Fe, Sn–Ni |
| Red | Si, Fe, Pb–K, Ca, Ti–Mn |
| Pink | Si, Pb–K, Ca, Fe, As–Au, Ni |
| Black | Si, K, Ca, Pb, Fe–Mn, Co, Cu–Ni, Zn |
| Period (Assignment Based on Decor Style) | Porcelain | Specific Technologies | Remarks | Refs |
|---|---|---|---|---|
| Final period of Kangxi reign (<1722) | G1710 vase | Ming blue H-red Sn-N.y.(s) | “Biyu tang zhi” mark Famille verte/Famille noire J.p.k. | [12] |
| G822 dish | Sn-N.y. (s) H-red | “Da Qing Kangxi nian zhi” mark Famille verte J.I.F | [12] | |
| G5696 bowl | Ming blue H-red Sn/Sb-N.y.(s) | “Da Qing Kangxi nian zhi” mark doucai J.I.F | [12] | |
| G5250 bowl | As-blue; Ming blue Sb-N.y.; Sn-N.y. Au° Cassiterite? | “Kangxi yu zhi” mark huafalang P.w. | [12,17] | |
| G3361 water dropper | As-blue As-white Sn-N.y. Au° | “Da Qing Kangxi nian zhi” mark Famille rose J.I.F. | [12] | |
| R1006 cup | Ming blue Sn-N.y. | This work | ||
| Yongzheng reign (1723–1735) | R1056 dish | As-white Sn-N.y. | This work | |
| R1041 cup | As-blue Au° | This work | ||
| MG4806 bowl | Ming blue Sb-N.y. (s) | “Yongzheng yu zhi” mark huafalang/yangcai J.I.F. | [12] | |
| MG913 bowl | As-blue As-white Sn-N.y. (s) H-red | “Yongzheng yu zhi” mark huafalang/yangcai J.I.F.(?) | [12] | |
| MG7368 bowl | Ming blue N.y. (s) H-red | “Da Qing Yongzheng nian zhi” mark, doucai J.p.k. | [12] | |
| R1175 dish | As-blue (A) CaF2(?), Au° Sn/Sb-N.y., H-red | This work | ||
| TH487 dish | As-blue Sn/Sb-N.y. Au° | This work | ||
| F1341C bottle | As-blue Sn-N.y. Au°; H-red | This work | ||
| SN284 milk pot | As-blue (A) Sn-N.y. Au° | This work | ||
| SN284 cup | Sn-N.y. Au° | This work | ||
| Qianlong reign (>1735–1750) | Shard bowl | As-blue As-white H-red Sn-N.y. (yellow) Sb-N.y. (green) | From Palace excavation | [13] |
| R1045 cup | As-blue | This work | ||
| F1429C teapot | As-blue(A) Sb-Sn-Y.y. Au° | This work | ||
| TH457 bottle | Ming blue Sn-N.y. Sb-Sn-N.y. Au°,H-red | This work | ||
| F1371C bottle | As-white As-blue (A) Sn-N.y. | This work | ||
| R1025 dish | As-blue (A) Au° | This work | ||
| R1048 cup | As-white, As-blue Sn-N.y., H-red | This work | ||
| Qianlong reign (1750–1800) | MG3668 teapot | As-blue, Au° As-white Sn-Sb-N.y. | Yixing p.k. | [12] |
| MG9604 teapot | As-blue As-white Sb-N.y. | Yixing p.k. | [12,17] |
| Period | Painted | Specific Technologies | Cloisonné | Specific Technologies | Remarks |
|---|---|---|---|---|---|
| Final period of Kangxi reign (<1722) | F1448C | Sb-Sn-N.y. Sn-N.y. Cassiterite Au° | Palace workshop? | ||
| Qianlong reign (1735-1750) | R957 | As-white As-blue As-blue (A) | Guangzhou | ||
| Qianlong reign (1750-1775) | Sn-N.y. | F1735C | Sb-Sn-N.y. Sn-N.y. | Qianlong mark | |
| F1440C | As-blue Sb-N.y. Sn-N.y. Au° | ||||
| Qianlong reign (<1775-1800) | R958 | As-blue (A) As-white Sn-N.y. Au° | Guangzhou | ||
| R975 | As-blue Sb-Sn-N.y. Au°? | Guangzhou | |||
| F1501 | As-blue Sb-Sn-N.y. Sn-N.y. Au°? | Palace workshop | |||
| F1467.1/.2 | Sb-N.y. Sn-N.y. Au° | Qianlong mark | |||
| F1467.1/.2 | As-blue Sb-N.y. Sn-N.y. Cassiterite | ||||
| Qianlong reign (>1800)? | F1698C | As-blue Sn-N.y. | Guangzhou |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B.; Yang, Y.; Droguet, V. Investigation of the Pigments and Glassy Matrix of Painted Enamelled Qing Dynasty Chinese Porcelains by Noninvasive On-Site Raman Microspectrometry. Heritage 2020, 3, 915-940. https://doi.org/10.3390/heritage3030050
Colomban P, Kırmızı B, Zhao B, Clais J-B, Yang Y, Droguet V. Investigation of the Pigments and Glassy Matrix of Painted Enamelled Qing Dynasty Chinese Porcelains by Noninvasive On-Site Raman Microspectrometry. Heritage. 2020; 3(3):915-940. https://doi.org/10.3390/heritage3030050
Chicago/Turabian StyleColomban, Philippe, Burcu Kırmızı, Bing Zhao, Jean-Baptiste Clais, Yong Yang, and Vincent Droguet. 2020. "Investigation of the Pigments and Glassy Matrix of Painted Enamelled Qing Dynasty Chinese Porcelains by Noninvasive On-Site Raman Microspectrometry" Heritage 3, no. 3: 915-940. https://doi.org/10.3390/heritage3030050